Applications of Boron tribromide
Nov 11,2019
Boron tribromide, BBr3, is a colorless, fuming liquid compound containing boron and bromine. Commercial samples usually are amber to red/brown, due to weak bromine contamination. It is decomposed by water and alcohols.BBr3 is highly Lewis acidic.
It coordinates to ethereal oxygens and promotes C–O bond cleavage to an alkyl bromide and an alkoxyborane that is hydrolyzed
to an alcohol during workup.
Applications
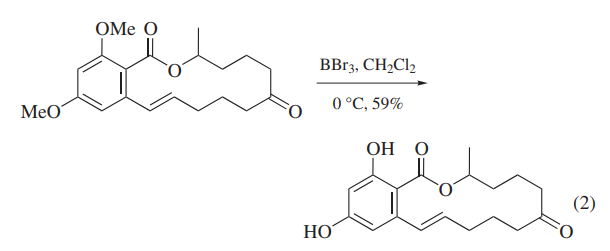
BBr3 has been widely used to cleave ethers because the reaction proceeds completely under mild conditions. In a special case, BBr3 has been used to cleave acetals that cannot be deprotected by usual acidic conditions.Because alkyl aryl ethers are cleaved at the alkyl–oxygen bond to give ArOH and alkyl bromides, BBr3 has been most generally used for the demethylation of methyl aryl ethers,for example as the final step of zearalenone synthesis (eq 2).Problems are sometimes encountered in attempts to deprotect more than one nonadjacent methoxy group on one aromatic ring, and when stable chelates are formed.6 The presence of a carbonyl substituent facilitates the selective deprotection of polymethoxyaryl compounds.
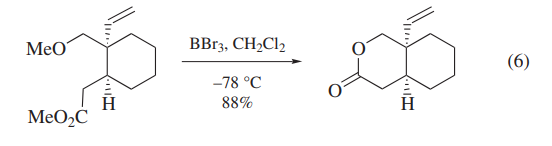
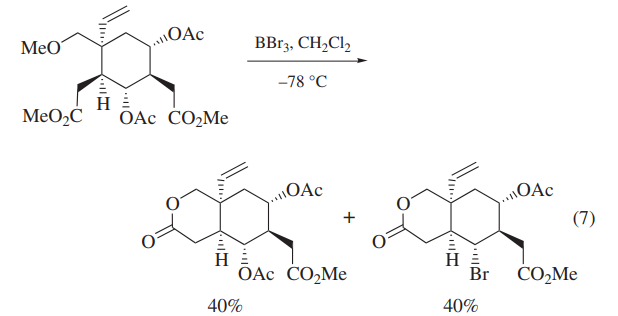
BBr3 has been also used for the deprotection of carbohydrate derivatives and polyoxygenated intermediates in the synthesis of deoxyvernolepin,vernolepin, and vernomenin.Although one of the model compounds is deprotected cleanly (eq 6),application of BBr3 to more highly functionalized intermediates leads to cleavage of undesired C–O bonds competitively (eq 7).
BBr3 can be used to effect cationic rearrangement reactions, for example, the Lewis acid-mediated ring-opening reaction and rearrangement of cyclobutyl ketones.The Lewis acid-mediated dimerization of 1,3-diarylpropargylic alcohols to give cyclobutane derivatives,the conversion of a trans-hexahydronaphthoxazine to the cis-isomer have all been achieved under the aegis of BBr3.
The BBr3-mediated conversion of 3-methoxypiperidines to 2-(bromomethyl)pyrrolidines, which proceeds via an intermediate bicyclic aziridinium ion, is an example of a rare conversion of piperidines into pyrrolidines.Renaud showed lactone ester can be obtained upon treatment of bridged bicyclic lactone with BBr3. This reaction consists of the simultaneous acidcatalyzed opening of the bicyclic ether and of the lactone.
Reference
1. McOmie, J. F. W.; Watts, M. L.; West, D. E., Tetrahedron 1968, 24,2289.
2. Meyers, A. I.; Nolen, R. L.; Collington, E. W.; Narwid, T. A.; Strickland,R. C., J. Org. Chem. 1973, 38, 1974.
3. (a) Benton, F. L.; Dillon, T. E., J. Am. Chem. Soc. 1942, 64, 1128.(b) Manson, D. L.; Musgrave, O. C., J. Chem. Soc. 1963, 1011.(c) McOmie, J. F. W.; Watts, M. L., Chem. Ind. (London) 1963, 1658.(d) Blatchly, J. M.; Gardner, D. V.; McOmie, J. F. W.; Watts, M. L., J.Chem. Soc. (C) 1968, 1545.
4. (a) Vlattas, I.; Harrison, I. T.; Tökés, L.; Fried, J. H.; Cross, A. D., J.Org. Chem. 1968, 33, 4176. (b) Taub, D.; Girotra, N. N.; Hoffsommer,R. D.; Kuo, C. H.; Slates, H. L.; Weber, S.; Wendler, N. L., Tetrahedron1968, 24, 2443.
5. (a) Stetter, H.; Wulff, C., Chem. Ber. 1960, 93, 1366. (b) Locksley, H.D.; Murray, I. G., J. Chem. Soc. (C) 1970, 392. (c) Bachelor, F. W.;Loman, A. A.; Snowdon, L. R., Synlett 1970, 48, 1554.
6. Schäfer, W.; Franck, B., Chem. Ber. 1966, 99, 160.
7. Bonner, T. G.; Bourne, E. J.; McNally, S., J. Chem. Soc. 1960, 2929.
8. Grieco, P. A.; Noguez, J. A.; Masaki, Y., J. Org. Chem. 1977, 42, 495.
9. Grieco, P. A.; Nishizawa, M.; Burke, S. D.; Marinovic, N., J. Am. Chem.Soc. 1976, 98, 1612.
10. (a) Grieco, P. A.; Hiroi, K.; Reap, J. J.; Noguez, J. A., J. Org. Chem.1975, 40, 1450. (b) Grieco, P. A.; Reap, J. J.; Noguez, J. A., Synth.Commun. 1975, 5, 155.
- Related articles
- Related Qustion
Vinyl acetate monomer (VAM) is a key intermediate used in the making of a number of polymers and resins for adhesives, coatings, paints, films, textiles and other end-products. Vinyl Acetate - VAM is used to make barrier resins for plastic....
Nov 11,2019Organic ChemistryZnS films were deposited onto glass substrates by the chemical bath technique at temperatures from 60 to 90°C. Zinc chloride, potassium hydroxide, ammonium nitrate, and thiourea were used as chemical components. According to the species dis....
Nov 11,2019Organic ChemistryBoron tribromide
10294-33-4You may like
- Rhenium:Discovery,Minerals,Chemistry,Uses,Toxicity
May 31, 2024
- How are Lead Minerals Distributed?
May 31, 2024
- Discovery and Major Minerals of Bismuth
May 31, 2024
Boron tribromide manufacturers
- Boron tribromide
-

- $10.00 / 10g
- 2024-04-15
- CAS:10294-33-4
- Min. Order: 10g
- Purity: 99
- Supply Ability: 100ton
- Boron tribromide
-

- $100.00 / 1bag
- 2023-09-13
- CAS:10294-33-4
- Min. Order: 1bag
- Purity: 99
- Supply Ability: 5000
- Boron tribromide
-

- $120.00 / 1KG
- 2023-08-16
- CAS:10294-33-4
- Min. Order: 1KG
- Purity: >99%
- Supply Ability: 50000kg/Month