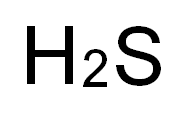How to form the Lewis structure and hybrid mode of H2S
Nov 24,2023
Lewis structure of H2S
The Lewis structure of H2S consists of a central sulphur atom (S) and two external hydrogen atoms (H) at a 92.1° degree bond angle. The sulphur atom (S) and the two hydrogen atoms (H) are each connected by a single bond. The Lewis structure of H2S is shown below:

Steps for drawing the H2S Lewis structure
Step 1 Calculate the number of valence electrons for S and H
Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom. So the total number of valence electrons in the H2S molecule = 1 valence electron from the sulphur atom + 2 valence electrons from the hydrogen atom = 6 + 1(2) = 8.
Step 2 Identify the central atom
The central atom must have a high valence or minimal electronegativity. For the H2S molecule, although hydrogen has a lower electronegativity than sulphur, the hydrogen atom needs only one electron to complete its valence shell layer; it has no d orbitals available to extend its valence shell, and therefore hydrogen is always placed on the outside if it is present in a given molecule. So sulphur is the central atom and hydrogen is the outer atom.
Step 3 Labelling the electron lone pairs between atoms
Total number of valence electron pairs = σ-bonds + π-bonds + lone pairs In the valence layer, the total number of electron pairs is obtained by dividing the total number of valence electrons by two. For the H2S molecule, the total number of electron pairs is 4. The sulphur atom is connected to each of the two hydrogen atoms by a σ-bond (one σ-bond equals one electron pair), and the remaining two electron pairs are distributed over the sulphur atom.
Step 4 Stability of the structure
In order for the central sulphur (S) atom to be stable, we must check that it has an octet. In the Lewis structure of hydrogen sulphide, the sulphur atom has two lone pairs of electrons and two bonded pairs of electrons, there are eight electrons which form an octet and therefore the sulphur atom is stable. Each hydrogen atom has two electrons around it and also reaches a steady state.
H2S hybridisation
In the H2S molecule, two hydrogen atoms form a σ-bond with the central sulphur atom. There are two single bonds in the molecule. These bonds occupy four valence electrons, so there are four valence electrons left. During bond formation, the s orbitals of the hydrogen atoms overlap the p orbitals of the sulphur atoms. The lone pair of electrons occupies two sp3 orbitals. the other two orbitals3 of sp overlap the 1s orbital of the hydrogen atom. Therefore, the hybridisation of the H2S molecule is sp3 hybridisation.
- Related articles
- Related Qustion
- The polarity of H2S and its uses Dec 21, 2023
Hydrogen sulfide is a colorless molecule with a chemical formula H2S. It is poisonous and has a foul odor like a rotten egg.
- Biological functions and Synthesis of Hydrogen sulfide Jun 24, 2022
Hydrogen sulfide is important in the regulation of many cellular processes and is strongly implicated in oxygen sensing.
- Hydrogen Sulfide-Hazard and Toxicity Sep 9, 2019
Hydrogen Sulfide is a colorless, very poisonous, flammable gas with the characteristic foul odor of rotten eggs. It often results from the bacterial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers where an
D-ribose is an aldehyde containing pentose sugar and is present in all living cells. It is an essential component for energy production in cells.....
Nov 24,2023Biochemical EngineeringL-Histidine is an essential amino acid used in the production of proteins and is frequently present at the active site of proteins.....
Nov 24,2023Biochemical EngineeringHydrogen Sulfide
7783-06-4You may like
Hydrogen Sulfide manufacturers
- HYDROGEN SULFIDE
-

- $15.00 / 1KG
- 2021-08-11
- CAS:7783-06-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton