Toxicity of Rifapentine
Mar 28,2022
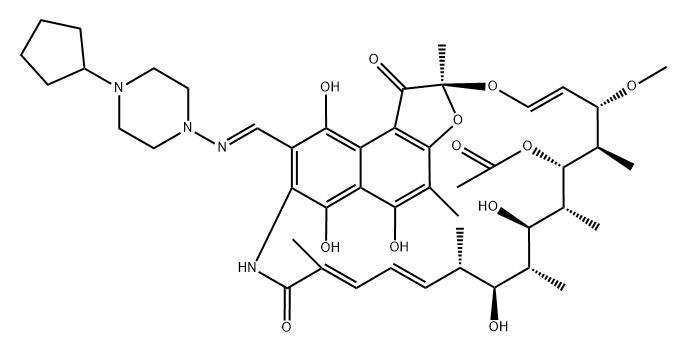
Rifapentine is a cyclopentyl-substituted rifampicin derivative, originally synthesized in 1965 and subsequently approved for use in the USA in 1998. It is bactericidal against Mycobacterium tuberculosis at low concentrations, by inhibiting the bacterial RNA polymerase and thus interfering with transcription of mRNA from DNA templates. The chemical formula of rifapentine, also known as cyclooentyl rifampin, is C47H64N4O12. The chemical structure is shown below.

Rifapentine is highly protein bound and possesses a long biological half-life, making it suitable for once-weekly dosing, which facilitates directly observed short-course antituberculous chemotherapy, although the optimal dosage and frequency of administration have yet to be determined. It is available as a 150-mg tablet sold under the trade name Priftins and a capsule formulation is also produced in China.
Mechanism
The mechanism of action is the same as rifampicin. Rifapentine forms a stable complex with the b-subunit of the bacterial DNA-dependent RNA polymerase with a high affinity, preventing transcription of mRNA.
Toxicity
The spectrum and toxicity of rifapentine is very similar to that reported for rifampicin. Rifapentine 600 mg weekly has been well tolerated in trials, with a discontinuation rate (3%) reportedly the same as for rifampicin-based chemotherapy. Approximately 2.5% of recipients developed an alanine aminotransferase (ALT) >5× normal compared with 3.5% in those receiving rifampicin, an insignificant difference. Studies using 900 or 1200 mg weekly have generally found good tolerability with treatment continuation rates equivalent to the 600- mg dose. The rate of hepatotoxicity (i.e. ALT (ALT) >5×normal) was 1% in patients receiving 900 mg of rifapentine combined with 900 mg isoniazid weekly for 12 weeks, and 4.3% in 47 patients who received 1200 mg of rifapentine and 900 mg isoniazid weekly for 16 weeks. Rifapentine may produce red–orange discoloration of body fluids as with rifampicin.
An immune-mediated hypersensitivity reported to occur in patients receiving rifampicin has not occurred in studies using 600 mg weekly dosing of rifapentine. However, a recent pharmacokinetic study described possible rifamycin hypersensitivity syndrome with an intense dosing regimen of 900 mg rifapentine three times weekly administered for seven doses, combined with daily moxifloxacin. One of 13 participants developed fever with hepatitis and another developed fever and rash, with both syndromes developing 2 days after the drug was ceased. Rifapentine displayed teratogenic effects in mice and rabbits and there is insufficient information regarding rifapentine’s safety in pregnancy to recommend its use. If pregnancy occurs in a woman receiving rifapentine, the drug should be ceased and rifampicin substituted.
- Related articles
- Related Qustion
Rifaximin (4-deoxy-4u-methylpyrido[1u,2u-1,2]imidazole-[5,4c]-rifamycin SV) is a synthetic antimicrobial and derivative of rifamycin. It was first described, as compound L-105, in 1982 and marketed in Italy in 1987. Its empirical formula is....
Mar 28,2022APIStreptomycin was the first antibiotic to be effective against tuberculosis and was the first drug of any kind to be tested in a randomized controlled trial. It is a natural product produced by a soil actinomycete and was isolated from Strep....
Mar 28,2022APIRifapentine
61379-65-5You may like
- Rifapentine
-

- $15.00/ kg
- 2024-04-24
- CAS:61379-65-5
- Min. Order: 1kg
- Purity: 99.912%
- Supply Ability: 10ton
- Rifapentine
-

- $0.00 / 25KG
- 2023-07-04
- CAS:61379-65-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Rifapentine
-

- $80.70 / 1Kg/Bag
- 2023-03-06
- CAS:61379-65-5
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 10000kg