1H-Indole-3-acetic acid, α-methyl-, methyl ester synthesis
- Product Name:1H-Indole-3-acetic acid, α-methyl-, methyl ester
- CAS Number:23304-43-0
- Molecular formula:C12H13NO2
- Molecular Weight:203.24
Yield:23304-43-0 36%
Reaction Conditions:
Stage #1: methyl 2-(1-acetyl-1H-indol-3-yl)acetatewith lithium dipropan-2-ylazanide in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2: iodomethane in tetrahydrofuran;hexane at -78 - 20; for 6.5 h;
References:
Fu, Beverly;Nazemi, Azadeh;Levin, Benjamin J.;Yang, Zhongyue;Kulik, Heather J.;Balskus, Emily P. [Journal of the American Chemical Society,2022,vol. 144,# 25,p. 11110 - 11119] Location in patent:supporting information
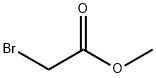
87-51-4
674 suppliers
$11.19/5G

1912-33-0
140 suppliers
$8.00/1g

58665-00-2
26 suppliers
$89.00/250mg
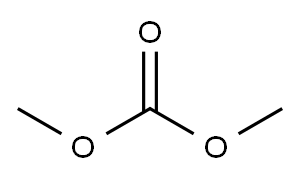
616-38-6
658 suppliers
$5.00/5G

23304-43-0
1 suppliers
inquiry

1912-33-0
140 suppliers
$8.00/1g

23304-43-0
1 suppliers
inquiry

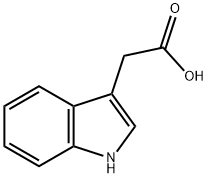
87-51-4
674 suppliers
$11.19/5G

23304-43-0
1 suppliers
inquiry