
2,2'-Azobis(2-methylpropionitrile) synthesis
- Product Name:2,2'-Azobis(2-methylpropionitrile)
- CAS Number:78-67-1
- Molecular formula:C8H12N4
- Molecular Weight:164.21

6869-07-4
13 suppliers
inquiry

78-67-1
399 suppliers
$24.73/1gm:
Yield:78-67-1 95%
Reaction Conditions:
with hydrogenchloride;12-molybdophosphoric acid hydrate;dihydrogen peroxide;sodium dioctyl sulfosuccinate;sodium bromide in lithium hydroxide monohydrate at 16 - 17; for 4.5 h;Reagent/catalyst;
Steps:
1 Combination of Reducing Agents with Phosphomolybdic Acid
A 1 liter glass reactor equipped with an anchor-type mechanical stirrer (stirring speed 500 rpm) is used. The reactor is equipped with a condenser. The hydrogen peroxide solution is introduced by means of a peristaltic pump via a flexible tube through the top of the reactor. The reactor is of jacketed type, cooled using a cryostat by circulation of cold water in the jacket. [0043] The cryostat bath is set at 13° C. and is circulated on the reactor. 116.8 g of DHC (0.610 mol), 300 mL of water, 42.5 g of aqueous HCl solution containing 5 mol/L of HCl (0.18 mol), 10 g of sodium bromide (0.097 mol) and 2.4 g of phosphomolybdic acid are placed in the reactor. 0.1 g of DOSS is added and stirring is started. When the temperature of the reactor stabilizes (14° C.), the pump for introducing the hydrogen peroxide solution is switched on. 62.2 g of a 34.5% H2O2 solution, i.e. 0.630 mol, are introduced, at a constant rate, over a period of 4 hours. The temperature of the medium stabilizes at 16-17° C. during the addition of H2O2. [0044] A sample is withdrawn at 1 hour of addition in order to check the consumption of the hydrogen peroxide introduced, and gives 0.18% of residual peroxide (if H2O2 were not consumed, about 1.4% of residual H2O2 solution would be obtained). [0045] At the end of addition of H2O2, the reaction mixture is left stirring for a further 30 minutes. It is then noted that the medium develops an orange-yellow color typical of the formation of bromine. In parallel, the temperature of the medium redescends, indicating the end of reaction. An equivalent peroxide assay is then performed and indicates 0.18%, corresponding to the free bromine and to the residual peroxide equivalents. [0046] The reaction mixture is then filtered through a sintered glass filter of porosity 4, and the mother liquors obtained, representing 402 g, are divided into 50 mL portions in glass flasks in order to perform the stability tests in the presence of the reducing agent. The flasks are stirred, and are then left to stand at room temperature (15-20° C.) for several days, in order to observe the formation of a deposit corresponding to the decomposition of the catalytic system. The results are collated in Table 1. [0047] The AZDN obtained is washed twice with 300 g of water, and 102.5 g of AZDN containing 9.2% water, i.e. a yield of 95%, are obtained.
References:
US2015/11738,2015,A1 Location in patent:Paragraph 0042-0047

19355-69-2
119 suppliers
$20.00/25g

78-67-1
399 suppliers
$24.73/1gm:

171915-82-5
2 suppliers
inquiry

78-67-1
399 suppliers
$24.73/1gm:
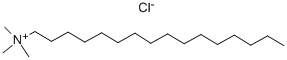
112-02-7
557 suppliers
$6.00/25g

19355-69-2
119 suppliers
$20.00/25g

78-67-1
399 suppliers
$24.73/1gm:

