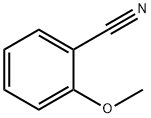
2-Methoxybenzonitrile synthesis
- Product Name:2-Methoxybenzonitrile
- CAS Number:6609-56-9
- Molecular formula:C8H7NO
- Molecular Weight:133.15
135-02-4
379 suppliers
$10.00/10g

6609-56-9
213 suppliers
$8.00/10g
Yield:6609-56-9 98%
Reaction Conditions:
with trifluorormethanesulfonic acid;trimethylsilylazide in acetonitrile at 25; for 0.00138889 h;Flow reactor;Schmidt Reaction;
Steps:
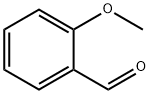
General procedure: General procedure for synthesis of nitriles from aldehydes incontinuous ow is as follows. Take benzaldehyde as an example,benzaldehyde (0.6 mmol) and TfOH (0.9 mmol) were dissolved in3 mL MeCN and pumped into inlet A. TMSN 3 (0.6 mmol) wasdissolved in 3 mL MeCN and pumped into inlet B (ow rate A:B = 54 m L/min:65 m L/min for 5 s residence time). The whole systemwas maintained at 25C. The ow system was equilibrated (about5 min), then the product stream was quenched and collected in aglass vessel with saturated aqueous NaClO in it. The ow systemwas stopped after the inlet A reduced 0.5 mL. The crude mixturewas dissolved in ethyl acetate and washed with saline solution. Thecombined organic layers were dried over magnesium sulfate,ltered, and concentrated under reduced pressure. The resultingcrude product was puried by ash chromatography on silica gelwith hexane and EtOAc as eluent to afford the product
References:
Zhan, Wei;Tong, Meng;Ji, Ling;Zhang, Han;Ge, Zemei;Wang, Xin;Li, Runtao [Chinese Chemical Letters,2019,vol. 30,# 5,p. 973 - 976]

29577-53-5
41 suppliers
$100.00/1g

6609-56-9
213 suppliers
$8.00/10g

612-16-8
215 suppliers
$11.00/5g

6609-56-9
213 suppliers
$8.00/10g

6850-57-3
217 suppliers
$10.00/1g

6609-56-9
213 suppliers
$8.00/10g

2439-77-2
86 suppliers
$25.00/1g

6609-56-9
213 suppliers
$8.00/10g