
3-METHYL-2-QUINOXALINOL synthesis
- Product Name:3-METHYL-2-QUINOXALINOL
- CAS Number:14003-34-0
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
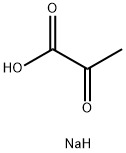
113-24-6
570 suppliers
$13.00/5g
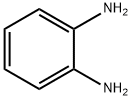
95-54-5
489 suppliers
$9.00/1g

14003-34-0
79 suppliers
$43.00/250mg
Yield: 92%
Reaction Conditions:
with acetic acid in water at 20; for 3 h;Reagent/catalyst;Temperature;
Steps:
2.1 Synthesis of 3-methyl-1H-quinoxaline-2-one (1)
In a round bottom flask equipped with a stirring bar was added o-phenylenediamine (0.20 g, 1.85 mmol) and sodium pyruvate (0.20 g, 1.85 mmol) in 25 mL aqueous acetic acid 20 %. The reaction was stirred at room temperature for 3 hours. After this time, the precipitate formed was collected by filtration and recrystallized from ethanol-water 4:1 v/v to afford pure product. Yield: 92 %; mp: 246-248 °C. 1H NMR (400 MHz, DMSO-d6): δ(ppm) 12.27 (br, 1H, NH), 7.68 (d, 1H, J = 7.8 Hz, Ar-H), 7.46 (t, 1H,J = 7.8 Hz, Ar-H), 7.25 (m, 2H, Ar-H), 2.40 (s, 3H, CH3) ; 13C NMR (100 MHz, DMSO-d6): δ(ppm) 159.6, 155.4, 132.4, 132.1, 129.7, 128.3, 123.4, 115.6, 20.9. Elemental analysis: Calcd: C, 67.49; H, 5.03; N, 17.49; Found: C, 67.47; H, 5.08; N, 17.51.
References:
da Costa, Erivaldo P.;Coelho, Sara E.;de Oliveira, André H.;Araújo, Renata M.;Cavalcanti, Livia N.;Domingos, Josiel B.;Menezes, Fabrício G. [Tetrahedron Letters,2018,vol. 59,# 44,p. 3961 - 3964] Location in patent:supporting information
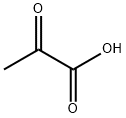
127-17-3
514 suppliers
$5.00/10g

95-54-5
489 suppliers
$9.00/1g

14003-34-0
79 suppliers
$43.00/250mg
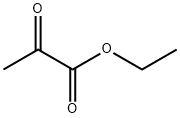
617-35-6
525 suppliers
$5.00/10g

95-54-5
489 suppliers
$9.00/1g

14003-34-0
79 suppliers
$43.00/250mg
75-91-2
159 suppliers
$26.00/100g

1196-57-2
317 suppliers
$6.00/10g

14003-34-0
79 suppliers
$43.00/250mg

1196-57-2
317 suppliers
$6.00/10g

3240-34-4
579 suppliers
$7.00/10g

14003-34-0
79 suppliers
$43.00/250mg