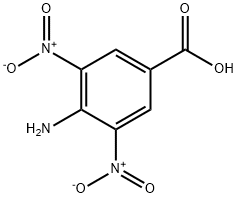
4-AMINO-3,5-DINITROBENZOIC ACID synthesis
- Product Name:4-AMINO-3,5-DINITROBENZOIC ACID
- CAS Number:7221-27-4
- Molecular formula:C7H5N3O6
- Molecular Weight:227.13

118-97-8
236 suppliers
$15.00/10g

7221-27-4
31 suppliers
inquiry
Yield:7221-27-4 98%
Reaction Conditions:
with ammonium hydroxide in methanol at 20; for 19.5 h;Reflux;
Steps:
5.1.2. 4-Amino-3,5-Dinitrobenzoic Acid (3)
Compound 2 (20 g, 81 mmol) was dissolved in methanol (100 mL), and aqueous 24% NH3 (120 mL)was gradually added. The reaction mixture was stirred at rt for 2.5 h, refluxed for 3 h and left atrt for around 14 h. The precipitate that formed was filtered o, and the filtrate was evaporated todryness. Water (10 mL) and HCl (10 mL) were added to the solid residue, which was combined withthe precipitate. After stirring for 10 min, the precipitate was filtered o and washed with water tillwashings showed neutral. The yield of 4-amino-3,5-dinitrobenzoic acid 3 was 18 g (98%) [16]. 1H NMR(300 MHz, Acetone-d6) = 9.16-9.01 (m, 3H), 9.01-8.84 (s, 2H). 13C NMR (75 MHz, Acetone-d6) 163.49, 143.33, 134.76, 133.75, 128.17, 115.39. HRMS (ESI): m/z calcd. for C7H4N3O6 [MH]: 226.0105;found, 226.0100.
References:
Aerschot, Arthur Van;Gadakh, Bharat;Lescrinier, Eveline;Nautiyal, Manesh;Pang, Luping;Rozenski, Jef;Strelkov, Sergei V.;Weeks, Stephen D.;Zhang, Baole;de Graef, Steff [Molecules,2020,vol. 25,# 20,art. no. 4751]

54321-79-8
52 suppliers
inquiry

7221-27-4
31 suppliers
inquiry
74-11-3
555 suppliers
$5.00/1g

7221-27-4
31 suppliers
inquiry

860698-40-4
0 suppliers
inquiry

7221-27-4
31 suppliers
inquiry

85365-92-0
4 suppliers
inquiry

7221-27-4
31 suppliers
inquiry