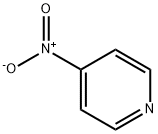
4-Nitropyridine synthesis
- Product Name:4-Nitropyridine
- CAS Number:1122-61-8
- Molecular formula:C5H4N2O2
- Molecular Weight:124.1
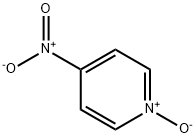
1124-33-0
334 suppliers
$10.00/1g

1122-61-8
99 suppliers
inquiry
Yield:1122-61-8 99%
Reaction Conditions:
with phosphorus trichloride in 1,2-dichloro-ethane;acetonitrile at 50; for 0.0833333 h;Flow reactor;Time;Temperature;
Steps:
Continuous flow procedure
At the same time, 1,2-dichloroethane (flow rate: 2.29 mLmin-1) is pumped into the extractor using another pump. After extraction,the 1,2-dichloroethane (DCE) phase containing 4-nitropyridine N-oxide(3) is injected through a pump to a second 37 mL tube coil PTFE(I.D.=2.1mm). Simultaneously, CH3CN (flow rate: 2.29 mL min-1) and thesolution of PCl3 (36.73 g, 0.267 mol, flow rate: 2.82 mL min-1) in 2454mL CH3CN are pumped into the PTFE tube using two separate pumps.The reduction reaction takes place in this second coil at 50 °C anda residence time of 5 min. The output flow of production from the coilcontinued directly into the product tank. After cooling, and then addingwater, the reaction mixture was made alkaline by addition of Na2CO3 andwas extracted with DCE. The DCE solution was dried over Na2SO4 andevaporated to dryness under reduced pressure. The two-step total yield of4-nitropyridine was 21.7 g (83%); m.p. 49-50 °C (lit.3 50 °C). 1H NMR(400 Hz, CDCl3): δ 8.92 (dd, J1 = 4.7Hz, J2 = 1.5 Hz, 2H), 8.01 (dd, J1 = 4.7Hz, J2 = 1.6 Hz, 2H).
References:
Wan, Zhidong;Fang, Zheng;Yang, Zhao;Liu, Chengkou;Gu, Jiajia;Guo, Kai [Journal of Chemical Research,2015,vol. 39,# 4,p. 209 - 212]

54750-97-9
0 suppliers
inquiry

1122-61-8
99 suppliers
inquiry
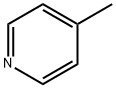
108-89-4
321 suppliers
$15.00/25mL

1122-61-8
99 suppliers
inquiry
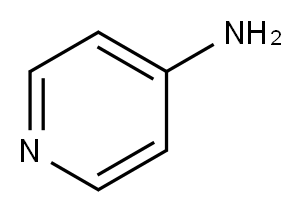
504-24-5
473 suppliers
$10.00/1 g

1122-61-8
99 suppliers
inquiry

1124-33-0
334 suppliers
$10.00/1g

1122-61-8
99 suppliers
inquiry
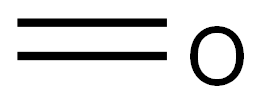
50-00-0
842 suppliers
$10.00/25g
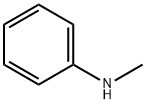
100-61-8
377 suppliers
$8.00/1g