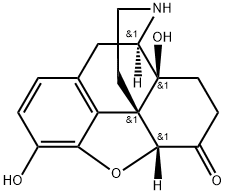
7,8-Dihydro-14-hydroxy- normorphinone synthesis
- Product Name:7,8-Dihydro-14-hydroxy- normorphinone
- CAS Number:33522-95-1
- Molecular formula:C16H17NO4
- Molecular Weight:287.32

76-41-5
2 suppliers
$16.00/1mg

33522-95-1
82 suppliers
$60.60/013-1ml
Yield:33522-95-1 79.7 %Chromat.
Reaction Conditions:
Stage #1:oxymorphone with di-isopropyl azodicarboxylate in water;N,N-dimethyl-formamide at 50; for 16 h;
Stage #2: with methanol;dimedone in N,N-dimethyl-formamide at 55 - 60; for 4 h;Product distribution / selectivity;
Steps:
EXAMPLES The following is an illustration of one way to carry out the invention. The Example is related to the demethylation of oxymorphone, however can also be performed for any conceivable compound reflected by formula (II).N-demethylation with an azodicarboxylate: Input: Content Amount in g Name of material mmol in % 50.0 Oxymorphone 154.893.3 w/w 233.0 Dimethyl formamide pure 62.6 Diisopropylazodicarboxylat (DIAD) 309.6 65.1 5,5-Dimethylcyclohexane-1,3-dione 464.3 (Dimedone) 19.8 Methanol pure 618.8 610.0 Dichlormethane 27.4 Hydrochloride acid approximately 32% 240.5 32 technical 450.0 Deionised water 26.2 Ammonia solution approximately 25%, 384.6 25 pure 120.0 Acetone 1664.1 Sum - - Output: Content Amount in g Name of material mol in % l 42.6 Noroxymorphone 130.3 87.9 287.31 Process: 1 50.0 g Oxymorphone are solved at room temperature in 233.0 g dimethylformamide at room temperature and supplemented with 62.6 g diisopropylazodicarboxylate. The solution is heated to 55° C. and a yellow to red mixture is formed. The solution is stirred for 4 hours at this temperature. 2 Progress of the reaction is controlled by HPLC for example. 3 65.1 g To the reaction mixture 19.8 g dimedone and methanol are added starting at 55° C. 4 The mixture is kept at a temperature of 60° C. whereas viscosity drops. The reaction mixture is stirred for 4 hours at a temperature of 60° C. 5 460.0 g The reaction mixture is kept at a temperature of 20° C. and is 200.0 g supplemented with 27.4 g dichlormethane, deionised water and hydrochloric acid 32% and is stirred for at least 5 min. Two clear phases are formed: a redish organic and a yellow aqueous phase. 6 The aqueous phase is separated. 7 150.0 g The aqueous phase is washed with dichlormethane. The phases are separated. 8 26.2 g To the aqueous phase aqueuos ammonia solution (25% w/w) are added at 20° C. under stirring. A suspension is formed. The further steps in the procedure comprise purification as used in chemistry. The steps described illustrate a possible way.Purification: 9 The suspension is brought to a temperature of 15° C. and stirred for at least two hours. 10 250.0 g The suspension is vacuum-filtered, dried and the residue is slurried with water at a temperature of 20° C. 11 The suspension is vacuum-filtered and dried well by aspiration. 12 100.0 g The filter residue is slurried with acetone at a temperature of 20° C., vacuum-filtered and is dried well by aspiration. 13 20.0 g The filter residue is again washed with acetone and well dried by aspiration. 14 The product is dried in a vacuum drying oven at 60° C. 15 42.6 g product is yielded as a fawn solid.As it can be derived from table 1, showing the influence of the solvents and the amount of DIAD on the yield of demethylated products, dimethylacetamide, dimethylformamide and mixtures of dimethylformamide/toluene brought about the highest reaction yield. Further, the amount of DIAD used preferably was in the range of 1.5 to 3.0 eq. TABLE 1 Noroxycodone: Examining the influence of the solvent and eq DIAD at 50° C. Data indicated as analysed by HLPC. conc. Solvent of eq HPLC Solvent System educt DIAD after Educt Product Toluene 10% 1.6 2.5 h 82.4% 11.1% 20 h 19.7% 61.4% Toluene 10% 2.5 2.5 h 72.2% 26.2% 20 h 1.7% 66.7% Toluene 10% 4.1 2.5 h 57.3% 38.3% 20 h 0.00% 79.8% Acetone 10% 1.6 2.5 h 72.1% 25.0% 20 h 11.0% 76.2% Acetone 10% 2.5 2.5 h 52.0% 42.3% 20 h 0.00% 86.0% Acetone 10% 4.1 2.5 h 36.0% 56.1% 20 h 1.2% 86.3% Methanol 10% 1.6 2.5 h 47.1% 20.3% 20 h 37.3% 8.5% Methanol 10% 2.5 2.5 h 3.0% 41.6% 20 h 1.5% 18.8% Methanol 10% 4.1 2.5 h 2.5% 50.4% 20 h 1.4% 20.0% Ethanol 10% 1.6 2.5 h 36.3% 38.5% 20 h 2.1% 31.8% Ethanol 10% 2.5 2.5 h 13.4% 56.0% 20 h 0.00% 26.0% Ethanol 10% 4.1 2.5 h 8.3% 63.2% MTBE 10% 3.0 16 h 26.5% 69.3% DMF 17% 3.0 16 h 1.0% 86.3% 42 h 1.6% 84.5% DMAc 17% 3.0 16 h 0.7% 91.0% 42 h 0.5% 92.5% ACN 17% 3.0 16 h 1.0% 80.0% EE 17% 3.0 16 h 3.5% 81.0% Toluene 33% 2.0 16 h 4.0% 69.0% Toluene 7% 2.0 16 h 28.0% 62.0% Toluene 33% 4.0 16 h 1.0% 71.0% 42 h 1.0% 58.0% Toluene 7% 4.0 16 h 7.0% 79.0% Toluene 20% 3.0 5 d 20° C. 2.0% 83.0% DMF 17% 3.0 17 h 1.0% 79.0% DMF 10% 3.0 17 h 1.0% 91.0% DMF 28% 3.0 17 h 1.0% 85.0% DMF 17% 2.0 17 h 1.0% 89.0% 1DMF 17% 1.5 17 h 1.0% 89.0% DMF/Toluene 6/1 (w/w) 15% 3.0 17 h 1.0% 88.0% DMF/Toluene 1/1 (w/w) 15% 3.0 17 h 1.0% 86.0% DMF/Toluene 1/6 (w/w) 15% 3.0 17 h 2.0% 85.0% DMF/Toluene 1/1 (w/w) 15% 1.5 16 h 0.0% 89.0% DMF/Toluene 1/1 (w/w) 15% 2.0 16 h 1.0% 89.0% DMF/Toluene 1/6 (w/w) 15% 1.5 16 h 11.0% 76.0% DMF/Toluene 1/6 (w/w) 15% 2.0 16 h 3.0% 83.0% DMF/Toluene 1/1 (w/w) 7% 1.5 16 h 4.0% 90.0% DMF/Toluene 1/1 (w/w) 7% 2.0 16 h 0.0% 92.0% DMF/Toluene 1/6 (w/w) 7% 1.5 16 h 23.0% 70.0% DMF/Toluene 1/6 (w/w) 7% 2.0 16 h 12.0% 81.0% DMF 15% 1.0 16 h 9.0% 83.0% Toluene dry 7% 2.0 20 h 5.0% 69.0% Toluene/water 95/5 7% 2.0 20 h 11.0% 62.0% (w/w) DMF/water 98/2 (w/w) 14% 2.0 16 h 0.7% 79.7% TABLE 2 Noroxycodone: Examining the influence of the solvent and eq DEAD at 50° C. Data indicated as analysed by HLPC. conc. of eq HPLC Solvent educt DEAD after Educt Product Toluene 10% 2.0 16 h 10.0% 64.0% TABLE 3 Noroxymorphone: Examining the influence of the solvent and eq DIAD at 50° C. Data indicated as analysed by HLPC. conc. of eq HPLC Solvent educt DIAD after Educt Product DMF 13% 2.0 17 h 4.6% 89.1% DMF 13% 2.0 16 h 1.5% 73.2%
References:
SIEGFRIED Ltd US2009/137809, 2009, A1 Location in patent:Page/Page column 3; 4; 5

84116-46-1
3 suppliers
inquiry

33522-95-1
82 suppliers
$60.60/013-1ml

64643-70-5
0 suppliers
inquiry

33522-95-1
82 suppliers
$60.60/013-1ml

465-65-6
62 suppliers
$27.89/004-1ml

33522-95-1
82 suppliers
$60.60/013-1ml

52446-24-9
3 suppliers
$690.00/250 mg

33522-95-1
82 suppliers
$60.60/013-1ml