
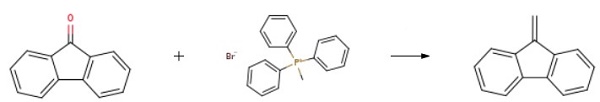
9-Methylene-9H-fluorene synthesis
- Product Name:9-Methylene-9H-fluorene
- CAS Number:4425-82-5
- Molecular formula:C14H10
- Molecular Weight:178.23
24324-17-2
481 suppliers
$6.00/25g

4425-82-5
66 suppliers
inquiry
Yield:4425-82-5 100%
Reaction Conditions:
with 1H-imidazole;PS-triphenylphosphine;iodine in dichloromethane;not specified; for 0.5 h;Heating / reflux;
Steps:
Compound 204a:; 204aTo a dichloromethane solution (35 imL) of triphenylphosphine (polymer-supported, 3 mmol/g, 1.87 g, 5.61 mmol) was added I2 (1.42 g, 5.61 mmol). The mixture was stirred for 15 minutes. Imidazole (410 mg, 6.38 mmol) was introduced and stirred gently at room temperature for another 15 minutes. 9- Fluorenyl methanol (500 mg, 2.55 mmole) was then introduced into the reaction mixture and refluxed for 30 minutes. It was filtered through a pad of celite. The filtrate was diluted with dichloromethane (30 mL), washed with saturated Na2S2O3 (30 mL), water (30 mL) and brine (30 nriL), dried with Na2SO4 and concentrated to give the title compound in 100% yield. 1H NMR (DMSO- d6): δ 7.88 (2H, d, J=7.3Hz), 7.83 (2H, d, J=7.6Hz), 7.41 (2H, td, J=7.6, 1.1 Hz), 7.33 (2H, td, J=7.6, 1.3Hz), 6.27 (2H, s). LCMS (APCI): 179.0 (M+H+).
References:
WO2006/40646,2006,A1 Location in patent:Page/Page column 130
![L-Histidine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-1-(triphenylmethyl)-, methyl ester](/CAS/20210305/GIF/937801-67-7.gif)






