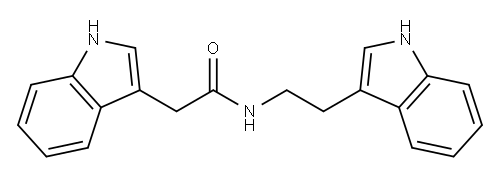
N-2-(indol-3-yl)ethyl-indole-3-acetamide synthesis
- Product Name:N-2-(indol-3-yl)ethyl-indole-3-acetamide
- CAS Number:82575-82-4
- Molecular formula:C20H19N3O
- Molecular Weight:317.38
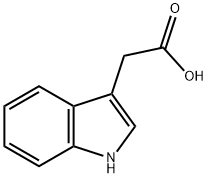
61-54-1
502 suppliers
$12.00/5G

82575-82-4
3 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 4 steps
1.1: 83 percent / Et3SiH / trifluoroacetic acid / 4 h / 68 - 71 °C
2.1: DCC; 1-hydroxy-1,2,3-benzotriazole / tetrahydrofuran / 1 h / 20 °C
2.2: 79 percent / tetrahydrofuran / 4.5 h / 20 °C
3.1: 55 percent / Na2WO4*2H2O; 30 percent H2O2 / methanol; H2O / 0.25 h / 20 °C
4.1: 8 percent / 85 percent HCOOH / H2O / 3 h / 20 °C
References:
Nakai, Yu-ya;Goto, Aya;Yamada, Fumio;Somei, Masanori [Heterocycles,2003,vol. 60,# 7,p. 1589 - 1600]

61-54-1
502 suppliers
$12.00/5G

50720-05-3
9 suppliers
$137.84/250mgs:

82575-82-4
3 suppliers
inquiry

13078-91-6
30 suppliers
$74.00/250mg

82575-82-4
3 suppliers
inquiry