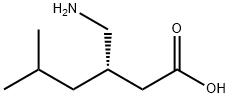
Pregabalin synthesis
- Product Name:Pregabalin
- CAS Number:148553-50-8
- Molecular formula:C8H17NO2
- Molecular Weight:159.23
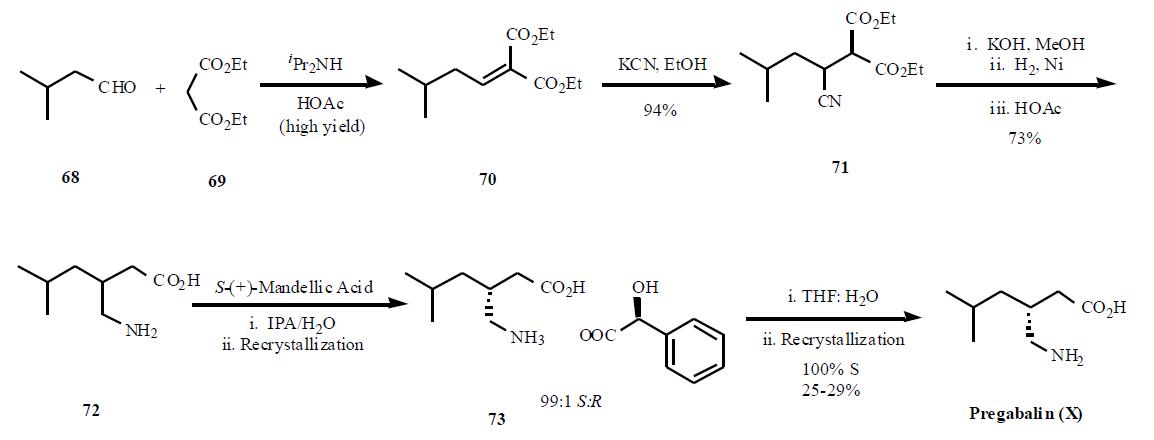
It’s worth noting that the Pfizer group have come up with a new process of preparing pregabalin (X) via enantioselective reduction, that promises to further reduce cost and waste associated with the manufacture of this drug.

157422-27-0
1 suppliers
inquiry

148553-50-8
765 suppliers
$39.00/1mg
Yield:148553-50-8 99%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol; for 3 h;
Steps:
7
Example 7: Preparation of pregabalin (Compound 1); Compound 4 (569.7 mg, 3.08 mmol) prepared in Example 5 and 10% Pd/C (90 mg) were added to methanol (30 mL) , and then stirred under a hydrogen balloon for 3 hrs. 10% Pd/C was removed by filtering with cellite, and the solvent was evaporated under reduced pressure to obtain Compound 1, pregabalin( (S) -3- (aminomethyl) -5-methylhexanoic acid) as a white solid (485 mg, 99.0%) .The obtained solid (Compound 1) had a melting point of 182
References:
WO2009/22839,2009,A2 Location in patent:Page/Page column 24-25

181289-45-2
2 suppliers
inquiry

148553-50-8
765 suppliers
$39.00/1mg

949890-75-9
3 suppliers
inquiry

148553-50-8
765 suppliers
$39.00/1mg

181289-39-4
57 suppliers
inquiry

148553-50-8
765 suppliers
$39.00/1mg

951792-40-8
1 suppliers
inquiry

148553-50-8
765 suppliers
$39.00/1mg