
PTZ-343 synthesis
- Product Name:PTZ-343
- CAS Number:101199-38-6
- Molecular formula:C15H16NNaO3S2
- Molecular Weight:345.41

1120-71-4
511 suppliers
$13.00/5g
92-84-2
525 suppliers
$10.00/5g

101199-38-6
152 suppliers
$19.00/250mg
Yield:101199-38-6 92.9%
Reaction Conditions:
Stage #1:10H-phenothiazine with sodium hydride in tetrahydrofuran;mineral oil at 20 - 50; for 1.5 h;Inert atmosphere;
Stage #2:1,3-propanesultone in tetrahydrofuran;mineral oil at 0 - 20; for 1 h;
Steps:
1 Synthesis of sodium 3-(phenothiazin-10-yl)propane-1-sulfonate
Example 1Synthesis of sodium 3-(phenothiazin-10-yl)propane-1-sulfonateA portion of sodium hydride (17.7 g, 0.44 moles) dispersed in mineral oil is first washed with petroleum ether (b.p. 40-60° C.) and then suspended in 200 mL dry tetrahydrofuran, in a 2 L three-neck flask. A solution of phenothiazine (80.0 g, 0.4 moles) dissolved in tetrahydrofuran (400 mL) is added under argon through a cannula. The mixture is shaken, always under argon, for one hour at room temperature and then for 30 minutes at 50° C. The mixture acquires an orange colour, following the formation of the phenothiazine anion. After the suspension has cooled to 0° C., a solution of 1,3-propanesultone (35 mL, 0.4 moles) in tetrahydrofuran is added also through a cannula. The colour of the mixture changes almost immediately to clear yellow. The mixture is stirred at 0° C. for thirty minutes after the addition of the sultone and then at room temperature for another thirty minutes.During this period, the mixture becomes almost colourless and homogeneous, with total dissolution of the material in suspension. Subsequently, the product precipitates in crystalline form. At the end of reaction, the product is separated from the solution by means of filtration and washed thoroughly first with tetrahydrofuran and then with ether. Yield: 127.6 g (92.9%). The product is already of high purity. In particular, chromatographic analysis (HPLC, λ=254 nm) shows that the content of phenothiazine in the product is less than 0.0002 parts (mole/mole). Other impurities can be removed efficiently by recrystallization from ethanol. Molecular mass (C15H14NaO3S2): 343.40. 1H NMR 300 MHz, D2O) δ: 6.9-7.1 (m, 4H, ArH), 6.7-6.9 (m, 4H, ArH), 3.8 (t, 2H, -CH2-N, J=9.9 Hz), 2.5 (t, 2H, -CH2-S, J=11.2 Hz), 1.8-2.0 (m, 2H, -CH2-CH2-CH2). Molecular mass of the free acid (C15H15O3S2): 321.42. MS (API-ES): 322.0 [MH]+.
References:
Cyanagen Srl US7855287, 2010, B2 Location in patent:Page/Page column 5

92357-95-4
12 suppliers
$650.00/50 mg

101199-38-6
152 suppliers
$19.00/250mg

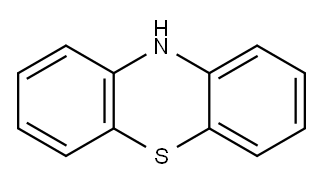
92-84-2
525 suppliers
$10.00/5g
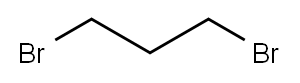
109-64-8
496 suppliers
$10.00/5g

101199-38-6
152 suppliers
$19.00/250mg