| Hazard Information | Back Directory | [Uses]
Antibacterial. | [Uses]
Sulfacytine is a soluble short-acting sulfonamide. Sulfacytine is used as an oral antibiotic. | [Definition]
ChEBI: Sulfacytine is a member of benzenes and a sulfonamide. | [Brand name]
Renoquid (Glenwood). | [Antimicrobial activity]
This drug is effective for infections caused by streptococci, gonococci, pneumococci, staphy�lococci, and also colon bacillus. Sulfacytine is used for pneumonia, cerebral meningitis,
staphylococcal and streptococcal sepsis, and other infectious diseases. A synonym of this
drug is renoquid. | [Synthesis]
Sulfacytine, N1-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)-sulfanilamide
(33.1.5), is synthesized by reacting 4-acetylaminobenzenesulfonyl chloride with 1-ethyl-cyto�sine (33.1.3) followed by reductive deacylation of the acetanilide part of the molecule (33.1.4)
using a system of zinc ¨C sodium hydroxide, which gives the desired sulfacytine.
1-Ethylcytosine (33.1.3) is in turn synthesized from 3-ethylaminopropionitrile, which is
reacted with cyanic acid (potassium cyanate¨Chydrochloric acid) in the first stage of synthesis
to give 1-(2-cyanoethyl)-1-ethylurea (33.1.1). This easily cyclizes to 1-ethyl-5,6-dihydrocyto�sine in the presence of sodium methoxide, and is isolated in the form of a hydrobromide
(33.1.2) for subsequent oxidation of the ordinary C5¨CC6 bond. Bromine turns out to be the
optimal oxidant for this purpose, and using nitrobenzene as the solvent gives a hetero�aromatic amine, 1-ethylcytosine (33.1.3), which was transformed to the desired sulfacytine in
the aforementioned manner?a by reacting it with 4-acetylaminobenzenesulfonyl chloride and
subsequent removal of the protecting acetyl group from the amine part of the molecule.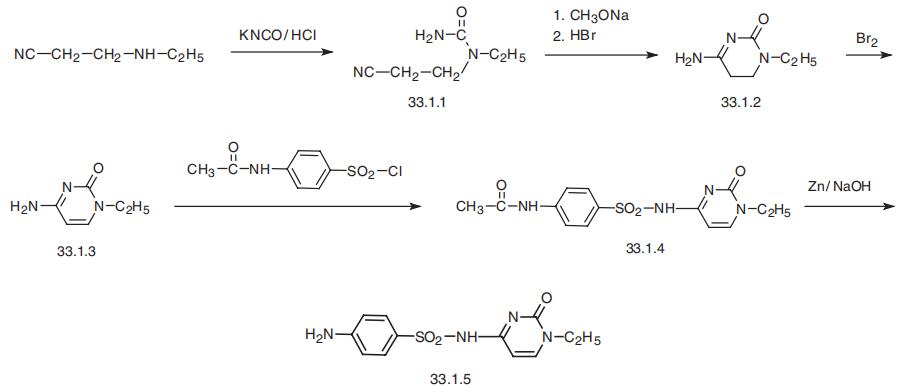
|
|
| Company Name: |
kemikalieimport
|
| Tel: |
+ 45 - 2034 3359 |
| Website: |
www.kemikalieimport.dk |
|