| Identification | Back Directory | [Name]
BENZENEACETIC ACID, 4-(2-(4-(1-(2-ETHOXYETHYL)-1H-BENZIMIDAZOL-2-YL)-1-PIPERIDINYL)ETHYL-ALPHA, ALPHA-DIMETHYL- | [CAS]
202189-78-4 | [Synonyms]
Bilatex
Bilaxten
Bilasten
Bilastin
Bilastine
the impurity of Bilastine
2-[4-[2-[4-[1-(2-Ethoxyethyl)benzimidazol-2-yl]piperidin-1-yl]ethyl]phenyl]-2-methylproionic acid
2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]piperidin-1-yl]ethyl]phenyl]-2-methylpropanoic acid
2-[4-[2-[4-[1-(2-ethoxyethyl)benzoimidazol-2-yl]-1-piperidyl]ethyl]phenyl]-2-methyl-propanoic acid
4-[2-[4-[1-(2-Ethoxyethyl)-1H-benziMidazol-2-yl]-1-piperidinyl]ethyl]-α,α-diMethylbenzeneacetic Acid
Benzeneacetic acid,4-[2-[4-[1-(2-ethoxyethyl)-1H-benziMidazol-2-yl]-1-piperidinyl]ethyl]-a,a-diMethyl-
Benzeneacetic acid, 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidinyl]ethyl]-α,α-dimethyl-
2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoic acid
4-[2-[4-[1-(2-Ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidinyl]ethyl]-alpha,alpha-dimethylbenzeneacetic acid
2-(4-(2-(4-(1-(2-ETHOXYETHYL)-1H-BENZO[D ]IMIDAZOL-2-YL) PIPERIDIN-1-YL)ETHY)PHEN YL)-2-METHYLPROPANOIC ACID
BENZENEACETIC ACID, 4-(2-(4-(1-(2-ETHOXYETHYL)-1H-BENZIMIDAZOL-2-YL)-1-PIPERIDINYL)ETHYL-ALPHA, ALPHA-DIMETHYL-
BENZENEACETIC ACID, 4-(2-(4-(1-(2-ETHOXYETHYL)-1H-BENZIMIDAZOL-2-YL)-1-PIPERIDINYL)ETHYL-ALPHA, ALPHA-DIMETHYL- USP/EP/BP | [Molecular Formula]
C28H37N3O3 | [MDL Number]
MFCD09837814 | [MOL File]
202189-78-4.mol | [Molecular Weight]
463.61 |
| Chemical Properties | Back Directory | [Melting point ]
202 °C | [Boiling point ]
639.1±55.0 °C(Predicted) | [density ]
1.16±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
DMSO:49.3(Max Conc. mg/mL);106.34(Max Conc. mM) | [Water Solubility ]
Insoluble in water | [form ]
powder to crystal | [pka]
4.40±0.10(Predicted) | [color ]
White to Light yellow |
| Hazard Information | Back Directory | [Description]
Bilastine, a potent and selective histamine H1 receptor antagonist, was
approved in Europe in 2010 for the treatment of allergic rhinoconjunctivitis
(AR) and urticaria (hives or skin rash). The original synthesis of bilastine involves alkylation of 2-piperidinyl-1H-benzimidazole with a phenethyltosylate,
the para position of which is substituted with a dimethyloxazoline
moiety serving as a masked carboxylic acid group. Alkylation of the
benzimidazole nitrogen with 2-chloroethyl ethyl ether followed by
unmasking of the oxazoline moiety with sulfuric acid provided bilastine.
In two major clinical trials, bilastine was effective
at relieving allergic rhinitis as assessed by measuring the severity of nasal (obstruction, rhinorrhea, itching, sneezing) and nonnasal (ocular
itching, tearing, ocular redness, itching of ears, and/or palate) symptoms. | [Originator]
FAES FARMA, S.A. (Spain) | [Uses]
Bilastine is a novel, nonsedating H1-antihistamine developed for symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria. | [Uses]
Labelled Bilastine. It is a novel, nonsedating H1-antihistamine developed for symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria. | [Definition]
ChEBI: Bilastine is a member of benzimidazoles. | [Brand name]
Bilaxten | [Clinical Use]
Bilastine is a selective histamine H1 antagonist approved for the
treatment of allergic rhinoconjunctivitis and urticaria (hives). This drug, which has proven to be well tolerated in toxicology profiling,
46 was discovered by the Spainsh firm FAES Farma and was
approved by the European Union in 2010. | [Synthesis]
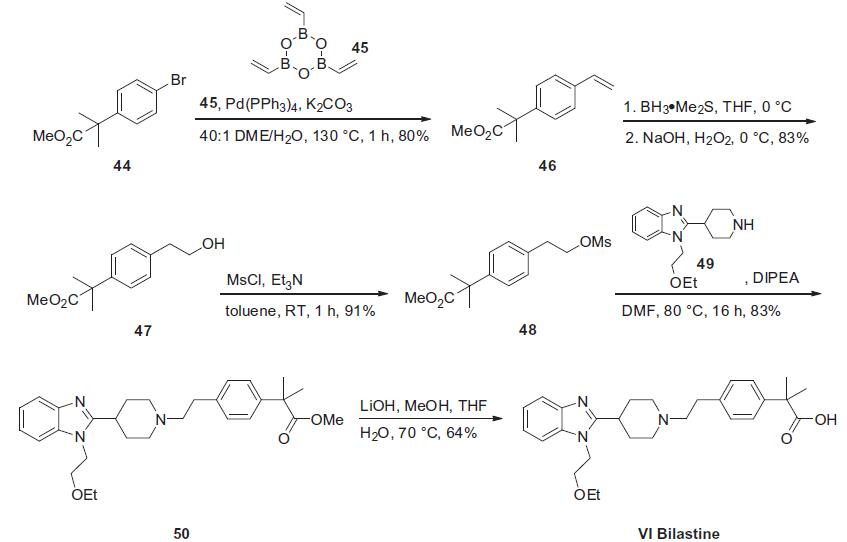
In 2011, Collier and
co-workers published a communication describing both the original
synthesis of bilastine and an improved route which was
amenable to gram-scale production. Collier’s second generation
route, shown below, relies upon a convergent approach
involving the union of piperidinyl benzimidazole 49 with fully
functionalized phenethyl electrophile 48.Coupling the commercially
available bromophenyl acetate 44 with cyclic trioxatriborinane
45 under conventional Suzuki conditions furnished styrene
46 in good yield. Alternatively, this vinylation reaction was also
performed under Stille conditions with tributyl vinyl stannane in
83% yield. Hydroboration–oxidation of 46 delivered phenethyl
alcohol 47 which was then immediately mesylated under basic
conditions in toluene to produce adduct 48. This sulfonate was
then reacted with piperidine 49 (whose preparation is described
in Scheme 7) followed by saponification of the resulting ester 50
to arrive at bilastene (VI) in 26% overall yield from 44.
For the preparation of bilastine piperidine 49,
commercially available piperidine 51 was first protected as the Boc-carbamate 52 prior to alkylation of the benzimidazole nitrogen
atom with 1-chloro-2-ethoxyethane 53, providing compound
54. The Boc group of 54 was removed under acidic conditions to
give fragment 49. This sequence produced the desired piperidine
component in 86% overall yield from 51.

| [Drug interactions]
Potentially hazardous interactions with other drugs
Antivirals: concentration possibly increased by
ritonavir.
Grapefruit juice: concentration of bilastine reduced. | [Metabolism]
Not significantly metabolised. Almost 95% of the
administered dose was recovered in urine (28.3%) and
faeces (66.5%) as unchanged bilastine |
|
|