| Identification | Back Directory | [Name]
(Z)-CAPSAICIN | [CAS]
25775-90-0 | [Synonyms]
civamide
Zucapsaicin
(Z)-CAPSAICIN
cis-capsaicin
cis-Capsaicine
(z)-6-nonenamid
Zucapsaicin,civaMide
(Z)-Capsaicin、Civamide
Civamide (Zucapsaicin)
Civamide cis-Capsaicin
Zucapsaicin(cis-Capsaicin)
(Z)-CAPSAICIN / Zucapsaicin
8-Methyl-N-vanillyl-6-nonenamide
(Z)-8-Methyl-N-vanillyl-6-nonenaMide
ZUCAPSAICIN;CIS-CAPSAICIN;CIVAMIDE;(Z)-CAPSAICIN
(Z)-N-(4-Hydroxy-3-Methoxybenzyl)-8-Methylnon-6-enaMide
(Z)-N-(4-Hydroxy-3-methoxybenzyl)-8-methyl-6-nonenamide
(Z)-8-Methyl-N-(4-hydroxy-3-methoxybenzyl)-6-noneneamide
(Z)-N-[(4-Hydoxy-3-methoxyphenyl)methyl]-8-metyl-6-nonenamide
(Z)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide
n-((4-hydroxy-3-methoxyphenyl)methyl)-8-methyl-6-nonenamide(z)-
(Z)-N-[(4-HYDROXY-3-METHOXYPHENYL)METHYL]-8-METHYL-6-NONENAMIDE
(6Z)-N-[(4-Hydroxy-3-Methoxyphenyl)Methyl]-8-Methyl-6-nonenaMide
6-Nonenamide, N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-, (6Z)- | [EINECS(EC#)]
636-760-0 | [Molecular Formula]
C18H27NO3 | [MDL Number]
MFCD00209942 | [MOL File]
25775-90-0.mol | [Molecular Weight]
305.41 |
| Chemical Properties | Back Directory | [Melting point ]
70 °C | [Boiling point ]
511.5±50.0 °C(Predicted) | [density ]
1.041±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
9.76±0.20(Predicted) | [color ]
White to Off-White | [Stability:]
Light Sensitive |
| Hazard Information | Back Directory | [Description]
Zucapsaicin is a topical analgesic that was approved in Canada in July
2010 for use in conjunction with oral COX-2 inhibitors or NSAIDs to
relieve severe pain in adults with osteoarthritis of the knee. Zucapsaicin
is the cis-isomer of the natural product capsaicin. Capsaicin is
available without a prescription in creams, lotions, and patches for the
treatment of neuropathic and musculoskeletal pain. Zucapsaicin is available
as a 0.075% by weight cream. The advantages of zucapsaicin compared
with capsaicin are reported to be a lesser degree of local irritation
(stinging, burning, erythema) in patients and a greater degree of efficacy
in preclinical animal models of pain. The analgesic action of
zucapsaicin and capsaicin is mediated through the transient receptor
potential vanilloid type 1 (TRPV1) channel. | [Originator]
E Merck AG (Germany) | [Uses]
(Z)-CAPSAICIN is used as a tool in neurobiological research. Prototype vanilloid receptor agonist.
| [Definition]
ChEBI: Zucapsaicin is a member of phenols and a member of methoxybenzenes. | [Brand name]
Civanex | [Clinical Use]
Zucapsaicin, the cis-isomer of the natural product capsaicin, is a
topical analgesic that was initially developed by Winston Pharmaceuticals
and approved in Canada in July 2010 for the treatment of
severe pain in adults with osteoarthritis of the knee. The advantages
of zucapsaicin compared with naturally-occurring capsaicin
are reported to be a lesser degree of local irritation (stinging, burning,
erythema) in patients and a greater degree of efficacy in preclinical
animal models of pain. The analgesic action of both
zucapsaicin and capsaicin is mediated through the transient receptor
potential vanilloid type 1 (TRPV1) channel, a ligand-gated ion
channel expressed in the spinal cord, brain, and localized on neurons
in sensory projections to the skin, muscles, joints, and
gut. | [Synthesis]
The scale preparation of zucapsaicin likely parallels the original
approach described by Gannett and co-workers involving the
coupling of vanillylamine with (Z)-8-methylnon-6-enoyl chloride.
216 Orito and co-workers elaborated this original approach in
an effort to prepare both capsaicin and zucapsaicin on gram-scale,
and this route is described in the scheme.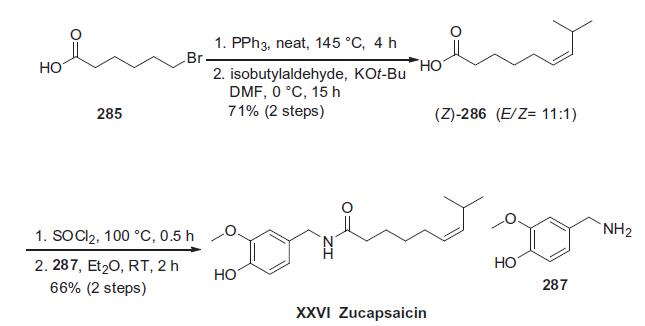
Commercial 6-bromohexanoic acid (285) was activated as the
Wittig salt prior to condensation with isobutylaldehyde in the
presence of strong base to generate an 11:1 ratio of E/Z-olefinic
acids favoring Z-isomer 286. Removal of the minor isomer was
easily achieved by short-path distillation.217 Interestingly, the
authors reported that facile olefin isomerization of 286 occurred
upon exposure to nitric acid at elevated temperatures, converting
286 to the corresponding E-isomer. Recrystallization provided
the product on multi-gram scale in 77% yield, representing a possible
scale production method for capsaicin. For the preparation
of zucapsaicin, acid 286 was converted the acid chloride via thionyl
chloride followed by immediate condensation with commercially
available vanillylamine (287). Two recrystallization steps were
subsequently employed to produce gram-scale amounts of zucapsaicin
(XXVI) in 66% yield overall for the two-step process. | [storage]
Store at -20°C,protect from light |
|
|