| Identification | Back Directory | [Name]
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide | [CAS]
290297-26-6 | [Synonyms]
Netupitan
CID6451149
Netupitant
Ro 67-31898
CID-6451149
CID 6451149
Ro 67-31898/000
Netupitant(CID-6451149)
NETUPITANT (RO 67-31898)
2-[3,5-Bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-y
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-
2-[3,5-Bis(trifluoromethyl)phenyl]-N-[6-(4-methylpiperazin-1-yl)-4-(o-tolyl)pyridin-3-yl]-N-methylisobutyramide
2-(3,5-bis(trifluoroMethyl)phenyl)-N,2-
diMethyl-N-(6-(4-Methylpiperazin-1-yl)-4-
o-tolylpyridin-3-yl)propanaMide
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
BenzeneacetaMide, N,a,a-triMethyl-N-[4-(2-Methylphenyl)-6-(4-Methyl-1-piperazinyl)-3-pyridinyl]-3,5-bis(trifluoroMethyl)-
Benzeneacetamide, N,α,α-trimethyl-N-[4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl]-3,5-bis(trifluoromethyl)- | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C30H32F6N4O | [MDL Number]
MFCD25976831 | [MOL File]
290297-26-6.mol | [Molecular Weight]
578.59 |
| Chemical Properties | Back Directory | [Melting point ]
156.2-160.0 °C | [Boiling point ]
597.4±50.0 °C(Predicted) | [density ]
1.255 | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
7.89±0.38(Predicted) | [color ]
White to Off-White | [InChIKey]
WAXQNWCZJDTGBU-UHFFFAOYSA-N | [SMILES]
C(N(C)C1=C(C2C=CC=CC=2C)C=C(N2CCN(C)CC2)N=C1)(=O)C(C1C=C(C(F)(F)F)C=C(C(F)(F)F)C=1)(C)C |
| Hazard Information | Back Directory | [Description]
Netupitant, originally developed by Helsinn Healthcare and
later licensed to Eisai, Inc., was approved in the USA in October
2014 for the treatment of chemotherapy-induced nausea and emesis.
Akynzeo ® is a fixed-dose combination of the new drug
netupitant and the previously-approved 5-HT3 antagonist palonosetron.
While palonosetron obtained approval previously for treating
nausea and emesis occurring within the first 24 hours (acute
phase) after chemotherapy, netupitant provides a synergistic
effect with palonosetron, assisting in prevention of nausea
and emesis in later stages following chemotherapy (25–120 h after
chemotherapy treatment). Several clinical trials showed that
this combination of netupitant and palonosetron (Akynzeo ?), in
comparison to treatment with palonosetron treatment alone, led
to an improved percentage of patients in all phases who did not
experience any nausea and emesis after undergoing chemotherapy. Netupitant itself joins the class of selective Neurokinin-
1 (NK1) receptor antagonists which, in addition to their use for treating chemotherapy-induced nausea and emesis,
also play an important role as therapies for depression and
anxiety. | [Uses]
Antiemetic. | [Definition]
ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 2-[3,5-bis(trifluoromethyl)phenyl]-2-methylpropanoic acid with the secondary amino group of N-methyl-4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin
3-amine; an antiemetic used in combination with palonosetron hydrochloride (under the trade name Akynzeo) to treat nausea and vomiting in patients undergoing cancer chemotherapy. | [Synthesis]
The most likely process-scale synthesis
of netupitant begins with 6-chloronicotinic acid (185). From
185, a one-pot 1,4-Grignard addition/oxidation reaction, developed
to provide an improved route to NK1 receptor antagonists, was
employed for direct installation of the C4-o-tolyl substituent. Using
this procedure, treatment of 6-chloronicotinic acid (185) with otolyl
magnesium chloride and subsequent oxidation with Mn
(OAc)2 in THF/AcOH generated the o-tolyl nicotinic acid intermediate
187 in 51% overall yield. From this intermediate, a one-pot
amide formation could be realized in high yield by conversion of
the acid to the corresponding acyl chloride and addition of NH4OH
(95% yield). Chloride displacement with 1-methyl piperazine under
heating conditions provided intermediate 189 in 95% yield.
Employing Hoffman reaction conditions originally reported by
Senanayake,171 rearrangement of amide 189 with NBS/NaOMe/
MeOH enabled formation of carbamate 190 in quantitative yield.
Reduction of the carbamate with Red-Al provided the desired
mono-methylated amine. To access the final drug target, acylation
of the intermediate methyl amine with 2-(3,5-bis(trifluoromethyl)
phenyl)-2-methylpropanoyl chloride (191) provided the final drug
netupitant (XXII) in 81% yield. In this case, due to the cost of 193,
the acid precursor to 191, and starting materials previously
reported for generating 191/193, as well as issues with isolation
of pure intermediates on scale, a novel route to 191 and 193 was
also developed during this synthesis, beginning with the inexpensive
and readily available bromide 192. This 2-step synthesis of 193 includes Grignard
reagent formation, quenching with acetone to yield the intermediary
tertiary alcohol, and subsequent carbonylation (TfOH, H2O, CO then NaOH/H2O) to provide 2-(3,5-bis(trifluoromethyl)-phenyl)-2-
methylpropanoic acid 193. Finally, conversion of acid 193 to the
acyl chloride with oxalyl chloride in DCM provided the necessary
acyl chloride 191 in quantitative yield (86% purity).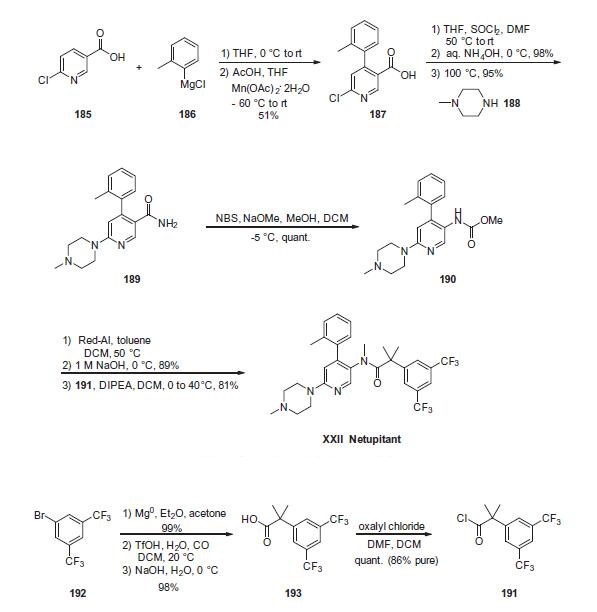
|
|
|