| Identification | Back Directory | [Name]
Pyrroloquinoline quinone | [CAS]
72909-34-3 | [Synonyms]
PQQ
PPQ
METHOXATIN
Methoxatine
Coenzime PQQ
Coenzyme PQQ
PQQ COFACTOR
PYRROLOQUINOLINE
PYRROLOQUINOLINE QUINONE
Pyrroloquinoline quinone ,98%
Pyrroloquinoline quinone(PQQ)
Methoxatin, Pyrroloquinoline, PQQ
Pyrroloquinoline Quinone Acid(PQQ)
PYRROLOQUINOLINE QUINONE, >=95.0% (H
PYRROLOQUINOLINE QUINONE, BIOCHEMIKA
4,5-dioxo-1h-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic Acid
4,5-Dihydro-4,5-dioxo-1H-pyrrolo[2,3-f]quinolin-2,7,9-tricarboxylic acid
4,5-Dioxo-4,5-dihydro-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid
4,5-DIHYDRO-4,5-DIOXO-1H-PYRROLO[2,3-F]QUINOLINE-2,7,9-TRICARBOXYLIC ACID
1H-Pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylicacid, 4,5-dihydro-4,5-dioxo-
PQQ Cofactor, Pyrroloquinoline quinone, 4,5-Dihydro-4,5-dioxo-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid
Pyrroloquinoline quinone,4,5-Dihydro-4,5-dioxo-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid, Methoxatin, PQQ | [EINECS(EC#)]
200-001-8 | [Molecular Formula]
C14H6N2O8 | [MDL Number]
MFCD00043125 | [MOL File]
72909-34-3.mol | [Molecular Weight]
330.21 |
| Chemical Properties | Back Directory | [Description]
Pyrroloquinoline quinone (PQQ) is a novel biofactor for which a proposition can be made for physiological importance. PQQ was first recognized as an enzyme cofactor in bacteria. It has recently been tentatively identified as a component of interstellar dust. Thus, PQQ may have been present throughout early biological conception and evolution. PQQ is also a potent plant growth factor. Consequently, for animals and humans, there has been constant exposure to PQQ. In animals, PQQ is reported to participate in a range of biological functions with apparent survival benefits (e.g., improved neonatal growth and reproductive performance). There are also benefits from PQQ supplementation related to cognitive, immune, and antioxidant functions, as well as protection from cardiac and neurological ischemic events. Although PQQ is not currently viewed as a vitamin, its involvement in cell signaling pathways, particularly those important to mitochondriogenesis in experimental animal models, may eventually provide a rationale for defining PQQ as vital to life. For humans, such evidence suggests there may be similar parallels or benefits from improving PQQ status. | [Appearance]
Orange-Red Solid | [Melting point ]
222 - 224°C | [Boiling point ]
1018.6±65.0 °C(Predicted) | [density ]
1.963±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
1.88±0.20(Predicted) | [color ]
Orange to Red | [BRN ]
3596812 | [Stability:]
Light and Temperature Sensitive | [InChI]
InChI=1S/C14H6N2O8/c17-10-4-2-6(14(23)24)15-8(4)7-3(12(19)20)1-5(13(21)22)16-9(7)11(10)18/h1-2,15H,(H,19,20)(H,21,22)(H,23,24) | [InChIKey]
MMXZSJMASHPLLR-UHFFFAOYSA-N | [SMILES]
N1C2=C(C3NC(C(O)=O)=CC=3C(=O)C2=O)C(C(O)=O)=CC=1C(O)=O | [Uses]
PQQ has been reported to function as a water soluble vitamin/cofactor and as an antioxidant. Pyrroloquinoline quinone (PQQ) disodium salt is proposed for use due to its nutritive value in the United States (U.S.) in foods, such as energy, sport, and isotonic drinks; non-milk based meal replacement beverages; water (bottled, enhanced, fortified); milk-based meal replacement beverages; cereal and granola bars; and energy, meal replacement, and fortified bars. PQQ is also intended for use in dietary supplements. |
| Hazard Information | Back Directory | [Chemical Properties]
Orange-Red Solid | [Uses]
A cofactor of microbial quinoprotein enzyme, and imidazopyrroline. A redox/cofactor found in a a class of enzymes called quinoproteins | [Application]
Pyrroloquinoline quinone has been used:
as a component of nanocurcumin formulation (NCF), to study its therapeutic effect on ameliorate hypoxia-induced stress in hypertrophied cardiomyocytes.
as a standard in fluorescence analysis.
to test its efficiency in suppressing restrained oxidative stress and hepatic fibrogenesis in mouse models.
Pyrroloquinoline quinone(PQQ) has been reported to function as a water soluble vitamin/cofactor and as an antioxidant. PQQ disodium salt is proposed for use due to its nutritive value in the United States (U.S.) in foods, such as energy, sport, and isotonic drinks; non-milk based meal replacement beverages; water (bottled, enhanced, fortified); milk-based meal replacement beverages; cereal and granola bars; and energy, meal replacement, and fortified bars. PQQ is also intended for use in dietary supplements. | [Definition]
ChEBI: Pyrroloquinoline quinone is a pyrroloquinoline having oxo groups at the 4- and 5-positions and carboxy groups at the 2-, 7- and 9-positions. It has a role as a water-soluble vitamin (role), a cofactor, an antioxidant and an anti-inflammatory agent. It is a member of orthoquinones, a tricarboxylic acid and a pyrroloquinoline cofactor. It is a conjugate acid of a pyrroloquinoline quinone(3-). | [General Description]
Pyrroloquinoline Quinone (PQQ), also referred as methoxatin, is a water soluble orthoquinone molecule with redox-cycling ability. | [Biochem/physiol Actions]
Pyrroloquinoline Quinone (PQQ) plays a vital role in gluconic acid production and biosynthesis by microbes. It acts as an effective microbial growth stimulant and biological control determinant for plant pathogens. PQQ also acts as an anti-melanogenic agent and is used to treat disorders related to hyper pigmentation. It exhibits diverse role in metabolism and cell signaling pathways. PQQ is used as a potential therapeutic to treat liver fibrosis. | [Synthesis]
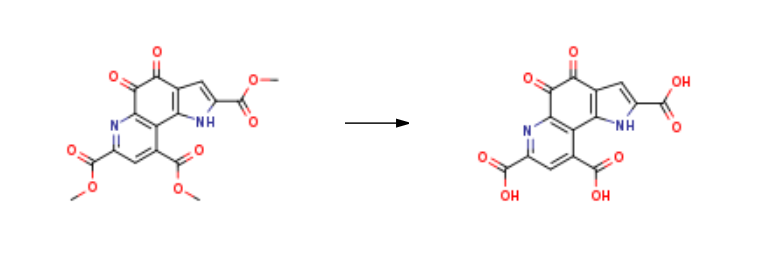
Pyrroloquinoline quinone is prepared by the reaction of
trimethyl 4,5-dihydro-4,5-dioxo-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylate. The steps are as follows:
With sodium carbonate In water at 30℃; for 24h;
Or with water; potassium carbonate In water at 25 - 80℃; Green chemistry; Industrial scale.
| [Purification Methods]
Efflorescent yellow-orange needles of PQQ are formed on recrystallising from H2O by addition of Me2CO, or better from a supersaturated aqueous solution, as it forms an acetone adduct. [Forrest et al. Nature 280 843 1979.] It has also been purified by passage through a C-18 reverse phase silica cartridge or a silanized silica gel column in aqueous solution whereby methoxantin remains behind as a red-orange band at the origin. This band is collected and washed thoroughly with dilute aqueous HCl (pH 2) and is then eluted with MeOH/H2O (7:3) and evaporated in vacuo to give the coenzyme as a red solid. It has also been purified by dissolving it in aqueous 0.5M K2CO3 and acidified to pH 2.5 whereby PQQ precipitates as a deep red solid which is collected and dried in vacuo. Methoxantin elutes at 3.55 retention volumes from a C18 _Bondapak column using H2O/MeOH (95:5) + 0.1% AcOH pH 4.5. It has UV max at 247 and 330nm (shoulder at 270nm) in H2O and max at 250 and 340nm in H2O at pH 2.5. With excitation at ex 365nm it has a max emission at 483nm. The 13C NMR has �: 113.86, 122.76, 125.97, 127.71, 130.68, 137.60, 144.63, 146.41, 147.62, 161.25, 165.48, 166.45, 173.30 and 180.00ppm. When a solution in 10% aqueous MeCO is adjusted to pH 9 with aqueous NH3 and kept at 25o for 30minutes, the acetone adduct is formed; UV has max at 250, 317 and 360nm (H2O, pH 5.5), and with ex at 360nm it has max fluorescence at max at 465nm; and the 13C NMR [(CD3)2SO, TMS] has �: 29.77, 51.06, 74.82, 111.96, 120.75, 121.13, 125.59, 126.88, 135.21, 139.19, 144.92, 161.01, 161.47, 165.17, 168.61, 190.16 and 207.03ppm. It also forms a methanol adduct. When it is reacted with Me2SO4/K2CO3 in dry Me2NCHO at 80o for 4hours, it forms the trimethyl ester which has m 265-267o(dec) [260-263o(dec) also reported] after recrystallisation from hot MeCN (orange crystals) with UV max at 252 and 344nm (H2O) and 251, 321 and 373nm (in MeOH; MeOH adduct ). [Duine et al. Eur J Biochem 108 187 1980, Duine et al. Adv Enzymology 59 169 1987, Corey & Tramontano J Am Chem Soc 103 5599 1981, Gainor & Weinreb J Org Chem 46 4319 1981, Hendrickson & de Vries J Org Chem 17 1148 1982, McKenzie et al. J Chem Soc, Chem Commun 1372 1983.] |
|
|