Apremilast
- CAS No.
- 608141-41-9
- Chemical Name:
- Apremilast
- Synonyms
- Apst;Apster;Apremilas;N-{2-[(1S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl}acetamide;CS-1055;Apr-im D;Apmisast;ApreMilast;Apremildst;The staples
- CBNumber:
- CB12563430
- Molecular Formula:
- C22H24N2O7S
- Molecular Weight:
- 460.5
- MDL Number:
- MFCD18782607
- MOL File:
- 608141-41-9.mol
- MSDS File:
- SDS
| Melting point | 152-156°C |
|---|---|
| Boiling point | 741.3±60.0 °C(Predicted) |
| Density | 1.381 |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly, Heated) |
| pka | 14.01±0.20(Predicted) |
| form | Solid |
| color | White to Pale Yellow |
| InChIKey | IMOZEMNVLZVGJZ-QGZVFWFLSA-N |
| SMILES | C(NC1=CC=CC2=C1C(=O)N([C@@H](C1=CC=C(OC)C(OCC)=C1)CS(C)(=O)=O)C2=O)(=O)C |
| CAS DataBase Reference | 608141-41-9 |
| FDA UNII | UP7QBP99PN |
| ATC code | L04AA32 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H361 | |||||||||
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P264-P270-P301+P312-P330-P501 | |||||||||
| NFPA 704 |
|
Apremilast price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 18502 | Apremilast ≥98% | 608141-41-9 | 1mg | $62 | 2024-03-01 | Buy |
| Cayman Chemical | 18502 | Apremilast ≥98% | 608141-41-9 | 5mg | $270 | 2024-03-01 | Buy |
| Cayman Chemical | 18502 | Apremilast ≥98% | 608141-41-9 | 10mg | $477 | 2024-03-01 | Buy |
| Cayman Chemical | 18502 | Apremilast ≥98% | 608141-41-9 | 25mg | $819 | 2024-03-01 | Buy |
| TRC | A729700 | Apremilast | 608141-41-9 | 2.5g | $825 | 2021-12-16 | Buy |
Apremilast Chemical Properties,Uses,Production
Description
Apremilast (Otezla) was approved by the FDA on March 21, 2014 for the treatment of adult patients with active psoriatic arthritis. Apremilast is a member of a new class of oral small molecule inhibitors of the phosphosphodiesterase 4 (PDE4) enzyme. When PDE4 is inhibited in immune cells, it results in elevation of intracellular cyclic adenosine monophosphate (cAMP) levels, which can regulate inflammatory mediators. The current clinical data indicate that apremilast is an effective and well tolerated option for the management of psoriasis and PsA in adults.
Uses
Treating active psoriatic arthritis. It is also used to treat moderate to severe plaque psoriasis in certain patients.
Metabolism and Drug interaction
Oral administration of apremilast has an absolute biovailability of 73% with peak concentration at 2.5 hours (Tmax). The half-life of apremilast is 6-9 hours. Apremilast is metabolized by the both cytochrome (CYP) and non-CYP pathways. In vitro studies have demonstrated that apremilast metabolism is primarily mediated by the CYP3A4 pathway. Thus, concomitant use of apremilast with CYP450 enzyme inducers (such as rifampin, phenobarbital, carbamazepine, phenytoin) is not recommended. There are no significant drug-drug interactions with oral contraceptives, ketoconazole, or methotrexate.
safety information
1.Apremilast may cause depression. The risk may be greater in patients who have a history of depression or suicidal thoughts or actions. Families and caregivers must closely watch patients who take apremilast. It is important to keep in close contact with the patient's doctor. Tell the doctor right away if the patient has new, worsened, or sudden symptoms, such as depression; anxiety, restlessness, or irritability; panic attacks; or any changes in behavior. Contact the doctor right away if any signs of suicidal thoughts or actions occur.
2.Apremilast may cause weight loss. You will need to have regular weight checks while you are taking apremilast.
side effects
Diarrhea; headache; nausea; weight loss.
References
1. http://www.rheumatology.org/Learning-Center/Publications-Communications/Drug-Safety/Hotline-Apremilast-for-the-Treatment-of-Psoriatic-Arthritis
2. https://www.ncbi.nlm.nih.gov/pubmed/26220911
3. https://www.drugs.com/cdi/apremilast.html
4. http://reference.medscape.com/drug/otezla-apremilast-999915
Description
Apremilast is the first and only oral phosphodiesterase IV (PDE- 4) inhibitor and anti-tumor necrosis factor alpha (TNFa) agent launched in the USA by Celgene for the treatment of active psoriatic arthritis (PsA). Apremilast was also approved for the treatment of moderate to severe plaque psoriasis in the USA. Later, this drug was also approved in the European Union (EU) for both indications. Although multiple approaches to the synthesis of apremilast have been described, the most likely process scale approach involves the construction of the challenging stereogenic benzylic carbon center by catalytic asymmetric hydrogenation.
Uses
Apremilast is an oral phosphodiesterase 4 inhibitor used in the treatment of adults with active psoriatic arthritis and adults with moderate to severe plaque psoriasis.
Definition
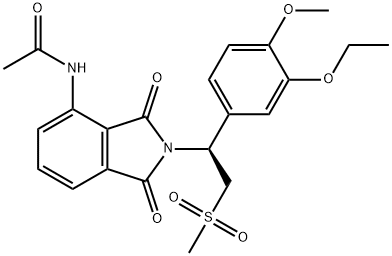
ChEBI: Apremilast is a member of the class of isoindoles that is isoindole-1,3-dione substituted at position 4 by an acetamido group and at position 1 by a 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl group. Used for treatment of psoriatic arthritis. It has a role as a phosphodiesterase IV inhibitor and a non-steroidal anti-inflammatory drug. It is an aromatic ether, a N-acetylarylamine, a sulfone and a member of phthalimides.
Clinical Use
Treatment of active psoriatic arthritis (PsA) and moderate to severe chronic plaque psoriasis (PSOR)
Synthesis
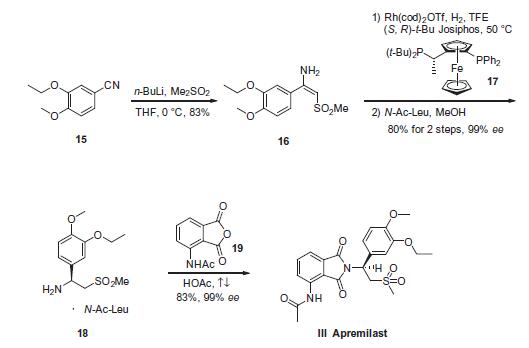
Dimethyl sulfone was first subjected to n-butyllithium in tetrahydrofuran (THF) prior to exposure to commercially available 3-ethoxy-4-methoxybenzonitrile (15) at low temperature to afford enamine 16 in 83% yield, presumably as a single isomer enamine possessing the E-configuration. After considerable studies conducted by researchers at Celgene regarding the reduction of this enamine and alternative substrates, enamine 16 was reduced under asymmetric hydrogenation conditions consisting of [Rh (COD)2]OTf and (S,R)-t-Bu Josiphos in trifluoroethanol (TFE) at 50 ?? under 90 psi of hydrogen pressure. Immediate exposure of the product to N-acetyl-L-leucine in methanol afforded the corresponding benzylamine salt 18 in 80% yield and more than 99% enantiomeric excess (ee). Finally, compound 18 was condensed with commercially available N-(phthalimid-3-yl)acetamide (19) in refluxing acetic acid to provide apremilast (III) in 83% yield and 99.4% ee.
Overdosage
Apremilast was studied in healthy subjects at a maximum total daily dose of 100 mg (given as 50 mg twice daily) for 4.5 days without evidence of dose limiting toxicities. In case of an overdose, it is recommended that the patient is monitored for any signs or symptoms of adverse effects and appropriate symptomatic treatment is instituted. In the event of overdose, symptomatic and supportive care is advised.
Enzyme inhibitor
This thalidomide-like psoriasis drug (FW = 460.50 g/mol; CAS 608141-41-9; Solubility: 90 mg/mL DMSO; <1 mg/mL H2O), also known as CC-10004, Otezla? and N-[2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methyl-sulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-1H-isoindol-4-yl]acetamide, is a potent, orally active phosphodiesterase inhibitor that targets phosphodiesterase PDE4 (IC50 = 74 nM), itself a proinflammatory mediator, and TNF-α, IC50 = 77 nM. Apremilast inhibits PBMC production of the chemokines CXCL9 and CXCL10, cytokines interferon-γ and tumor necrosis factor-α (TNF-α), and interleukins IL-2, IL-12 and IL- 23 from human rheumatoid synovial membrane cultures. Apremilast significantly reduces clinical scores in both murine models of arthritis over a ten-day treatment period and maintains healthy joint architecture in a dose-dependent manner. Unlike rolipram, however, apremilast demonstrated no adverse behavioral effects in na?ve mice. Otezla is specifically indicated by the Food & Drug Administration for the treatment of patients with moderate to severe plaque psoriasis who are typically candidates for phototherapy or systemic therapy. That said, the specific mechanism(s) for therapeutic action in psoriatic arthritis patients and psoriasis patients is unclear.
target
PDE4
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone - avoid.
Metabolism
Apremilast is extensively metabolised by both CYP and non-CYP mediated pathways including oxidation, hydrolysis, and conjugation, suggesting inhibition of a single clearance pathway. After oral administration of radiolabelled apremilast, about 3% and 7% of the radioactive dose is recovered as apremilast in urine and faeces, respectively.
Mode of action
Apremilast, an oral small-molecule inhibitor of phosphodiesterase 4 (PDE4), works intracellularly to modulate a network of pro-inflammatory and anti-inflammatory mediators. PDE4 is a cyclic adenosine monophosphate (cAMP)-specific PDE and the dominant PDE in inflammatory cells. PDE4 inhibition elevates intracellular cAMP levels, which in turn down-regulates the inflammatory response by modulating the expression of TNF-α, IL-23, IL-17 and other inflammatory cytokines. Cyclic AMP also modulates levels of anti-inflammatory cytokines such as IL-10. These pro- and anti-inflammatory mediators have been implicated in psoriatic arthritis and psoriasis.


Apremilast Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| HAINAN POLY PHARMCEUTICAL CO. LTD | +86-0571-89385087 +8613616530509 | ff@hnpoly.com | China | 118 | 58 |
| Beijing Mesochem Technology Co.,Ltd | +8613651027935 | rachel@mesochem.com | China | 191 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12471 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 18226 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 371 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 3001 | 58 |
Related articles
- Apremilast Uses and Relevance to the Military
- Apremilast effectively treats psoriasis and psoriatic arthritis, offering advantages for military personnel in austere conditi....
- Jan 29,2024
- Apremilast: A Review in Psoriasis and Psoriatic Arthritis
- Apremilast (Otezla) is an orally administered, small molecule inhibitor of phosphodiesterase 4 (PDE4). Apremilast 30 mg twice ....
- Feb 24,2023
View Lastest Price from Apremilast manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-21 | Apremilast
608141-41-9
|
US $0.00-0.00 / KG | 1KG | 99 | 50000KG/month | Shanghai Affida new material science and technology center | |
 |
2024-05-20 | Apremilast
608141-41-9
|
US $0.00 / kg | 1kg | 98% HPLC | 1000 kgs | PUSHAN INDUSTRIAL (SHAANXI) CO.,LTD | |
 |
2024-05-08 | Apremilast
608141-41-9
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up | 20 tons | Sinoway Industrial co., ltd. |
-

- Apremilast
608141-41-9
- US $0.00-0.00 / KG
- 99
- Shanghai Affida new material science and technology center
-

- Apremilast
608141-41-9
- US $0.00 / kg
- 98% HPLC
- PUSHAN INDUSTRIAL (SHAANXI) CO.,LTD
-

- Apremilast
608141-41-9
- US $0.00 / Kg/Bag
- 99% up
- Sinoway Industrial co., ltd.
608141-41-9(Apremilast)Related Search:
1of4





