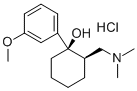
Tramadol hydrochloride
- CAS No.
- 22204-88-2
- Chemical Name:
- Tramadol hydrochloride
- Synonyms
- Amadol;TRAMODOL HCL;TRAMADOL HCL;AURORA KA-863;cis-Tramadol HCl;TRAMADOL HYDROCHLORIDE;CIS-TRAMADOL HYDROCHLORIDE;O-DESMETHYL-CIS-TRAMADOL HCL;tramadol hydrochloride tablets;cis-Tramadol hydrochloride solution
- CBNumber:
- CB4104737
- Molecular Formula:
- C16H26ClNO2
- Molecular Weight:
- 299.84
- MDL Number:
- MFCD00798507
- MOL File:
- 22204-88-2.mol
| Melting point | 171°C |
|---|---|
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| BCS Class | 1 |
| CAS DataBase Reference | 22204-88-2(CAS DataBase Reference) |
| FDA UNII | 9N7R477WCK |
| NCI Dictionary of Cancer Terms | tramadol hydrochloride |
| NCI Drug Dictionary | tramadol hydrochloride |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H225-H301+H311+H331-H370 |
| Precautionary statements | P210-P260-P280-P301+P310+P330-P308+P311-P403+P233 |
| Hazard Codes | F,T |
| Risk Statements | 11-23/24/25-39/23/24/25 |
| Safety Statements | 16-36/37-45 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 2 |
| Toxicity | LD50 in mice, rats (mg/kg): 350, 228 orally; 200, 286 s.c. (Lagler) |
Tramadol hydrochloride Chemical Properties,Uses,Production
Chemical Properties
White Cyrstalline Solid
Originator
Tramadol,Gruenenthal,W. Germany,1977
Uses
An Analgesic
Definition
ChEBI: (R,R)-tramadol hydrochloride is a hydrochloride resulting from the reaction of (R,R)-tramadol with 1 molar equivalent of hydrogen chloride; the (R,R)-enantiomer of the racemic opioid analgesic tramadol hydrochloride, it exhibits ten-fold higher analgesic potency than the (S,S)-enantiomer. It has a role as a delta-opioid receptor agonist, a kappa-opioid receptor agonist, a mu-opioid receptor agonist, an adrenergic uptake inhibitor, an antitussive, a capsaicin receptor antagonist, a muscarinic antagonist, a nicotinic antagonist, a NMDA receptor antagonist, an opioid analgesic, a serotonergic antagonist and a serotonin uptake inhibitor. It contains a (R,R)-tramadol(1+). It is an enantiomer of a (S,S)-tramadol hydrochloride.
Manufacturing Process
5 g of magnesium turnings are treated while stirring with a mixture of 37.4 g
of m-bromoanisole and 160 ml of absolute tetrahydrofuran at such a rate that
the reaction mixture boils gently because of the heat produced by the
immediately starting reaction. Thereafter, the reaction mixture is boiled under
reflux while stirring until all the magnesium dissolves. The reaction mixture is
cooled to 0°C to -10°C and then a mixture of 23.25 g of 2-
dimethylaminomethylcyclohexanone and 45 ml of absolute tetrahydrofuran is
added dropwise.
The resulting mixture is stirred for 4 hours at room temperature and then
poured, while stirring slowly, into a mixture of 25 g of ammonium chloride, 50
ml of water and 50 g of ice. The layers are separated and the aqueous layer is
extracted twice with 50 ml portions of ether. The organic layers are combined,
dried with sodium sulfate and evaporated. The residue is distilled, and 1-(m_x0002_methoxyphenyl)-2-dimethylaminomethyl-cyclohexanol-(1), boiling point at 0.6
mm Hg 138°C to 140°C, is obtained in a yield of 78.6% of theoretical. The hydrochloride obtained from the product, e.g., by dissolving in ether and
treating with dry hydrogen chloride, melts at 168°C to 175°C. By
recrystallization from moist dioxan this hydrochloride is separated into isomers
melting at 162°C to 163°C and 175°C to 177°C, respectively.
Therapeutic Function
Analgesic
Clinical Use
Analgesic
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: possible opioid withdrawal with
buprenorphine and pentazocine.
Anticoagulants: enhances effect of coumarins.
Antidepressants: possibly increased serotonergic
effects with duloxetine, mirtazapine or venlafaxine;
possible CNS excitation or depression with MAOIs
and moclobemide - avoid with MAOIs as increased
risk of serotonergic effects and convulsions; increased
risk of CNS toxicity with SSRIs or tricyclics.
Antiepileptics: effect reduced by carbamazepine.
Antihistamines: increased sedative effects with
sedating antihistamines.
Antipsychotics: enhanced hypotensive and sedative
effects; increased risk of convulsions.
Atomoxetine: increased risk of convulsions.
Dapoxetine: possible increased risk of serotonergic
effects - avoid.
Dopaminergics: avoid with selegiline.
Nalmefene: avoid concomitant use.
Sodium oxybate: enhanced effect of sodium oxybate
- avoid concomitant use.
Metabolism
Tramadol is metabolised by N- and O-demethylation via the cytochrome P450 isoenzymes CYP3A4 and CYP2D6 and glucuronidation or sulfation in the liver. Only O-desmethyl-tramadol is pharmacologically active. Tramadol and its metabolites are almost completely excreted renally.