ALAMETHICINRESEARCH GRADE
- CAS No.
- 27061-78-5
- Chemical Name:
- ALAMETHICINRESEARCH GRADE
- Synonyms
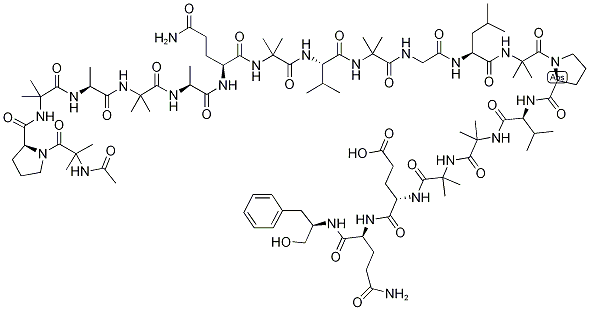
- Alamethicin (U-22324);ALAMETHICINRESEARCH GRADE;Alamethicin, Ready Made Solution;alamethicin from trichoderma viride;Alamethicin, Ready Made Solution from Trichoderma viride;Peptaibol coMplex. Additional synonyMs for the Major coMponent, alaMethicin I: AlaMethicin F30, AlaMethicin-gaMMa, U 22324;Ac-2-MeAla-L-Pro-2-MeAla-L-Ala-2-MeAla-L-Ala-L-Glu(NH2)-2-MeAla-L-Val-2-MeAla-Gly-L-Leu-2-MeAla-L-Pro-L-Val-2-MeAla-2-MeAla-L-Glu-L-Glu(NH2)-phenylalaninol;Almethicin, Antibiotic U-22324, 6-(2-methylalanine)-8-L-leucine-9-de-L-valine-12-(2-methylalanine)-13a-endo-(2-methylalanine)-18-L-glutamine-19-(N(1)-(1-(hydroxymethyl)-3-methylbutyl)-L-glutamamide)-];N-Acetyl-2-methylalanyl-L-prolyl-2-methylalanyl-L-alanyl-2-methylalanyl-L-alanyl-L-glutaminyl-2-methylalanyl-L-valyl-2-methylalanylglycyl-L-leucyl-2-methylalanyl-L-prolyl-L-valyl-2-methylalanyl-2-methylalanyl-L-α-glutamyl-N1;(3S,12R)-1-((S)-1-((6S,12S,15S,21S,30S)-1-((R)-1-(2-AcetaMido-2-Methylpropanoyl)pyrrolidin-2-yl)-15-(3-aMino-3-oxopropyl)-30-isobutyl-21-isopropyl-3,3,6,9,9,2,18,18,24,24,33,33-dodecaMethyl-1,4,7,10,13,16,19,22,25,28,31-undecaoxo-2,5,8,11,1
- CBNumber:
- CB4500700
- Molecular Formula:
- C92H150N22O25
- Molecular Weight:
- 1964.3078
- MDL Number:
- MFCD00151517
- MOL File:
- 27061-78-5.mol
- MSDS File:
- SDS
| Flash point | 87℃ |
|---|---|
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Methanol (up to 10 mg/ml). |
| form | DMSO solution |
| color | Off-white |
| BRN | 5213858 |
| Stability | Stable. |
| FDA UNII | 0LT1I1B7S8 |
ALAMETHICINRESEARCH GRADE price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A4665 | Alamethicin from Trichoderma viride ≥98% (HPLC) | 27061-78-5 | 5mg | $299 | 2024-03-01 | Buy |
| Sigma-Aldrich | A4665 | Alamethicin from Trichoderma viride ≥98% (HPLC) | 27061-78-5 | 10mg | $510 | 2024-03-01 | Buy |
| Sigma-Aldrich | A5361 | Alamethicin, Ready Made Solution from Trichoderma viride 5?mg/mL in DMSO, sterile; 0.2 μm filtered | 27061-78-5 | 500μl | $286 | 2024-03-01 | Buy |
| Cayman Chemical | 11425 | Alamethicin ≥90% | 27061-78-5 | 1mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 11425 | Alamethicin ≥90% | 27061-78-5 | 5mg | $117 | 2024-03-01 | Buy |
ALAMETHICINRESEARCH GRADE Chemical Properties,Uses,Production
Description
Alamethicin is an antibiotic peptide belonging to a class of membrane active peptides of fungal origin called peptaibols. It contains an unusual amphiphilic amino acid, 2-
Chemical Properties
solid
Uses
Alamethicin, Ready Made Solution from Trichoderma viride has been used :
- In the uridine 5′-diphospho-glucuronosyltransferase activity assay.
- To determine the Na, K-ATPase activity in permeabilized bovine nonpigmented epithelium cells.
- In methylcrotonyl-CoA carboxylase activity assay.
Uses
Alamethicin is a monovalent cation ionophore which can mimic nerve action potential across artificial membranes. Alamethicin has also been used for studying membrane interactions of antimicrobial peptides.
Uses
Alamethicin is an acidic linear peptaibol complex with potent antibiotic activity, containing 20 “amino acids”, with acetyl and phenylalaninol termini, produced by Trichoderma sp.. Alamethicin F30 acts an ionopohore, transporting ions through membranes and artificial lipid membranes and forming voltage-dependent ion channels in lipid bilayer membranes. Alamethicin is co-produced with a parallel, neutral, linear peptaibol complex (alamethicin F50). The relative composition of alamethicin is a variable mixture of the acidic and neutral complexes, but reports on alamethacin do not generally specify the ratio of F50 to F30, and comparative data between the complexes is scant.
General Description
Alamethicin belongs to the peptaibol family of antimicrobial peptides. It is mainly composed of hydrophobic amino acids. It possess a helical structure with a movable hinge region at Gly-11 position.
Biochem/physiol Actions
Alamethicin is a 20-amino acid channel-forming peptide antibiotic isolated from the fungus Trichoderma viride. Alamethicin consists of several isoforms, for which structural information has been published. Alamethicin forms voltage-dependent channels across lipid bilayer membranes. The Alamethicin channel is built by a bundle of helical monomers forming a water filled transmembrane pore. The conductivity level of the channel is determined by the number of associated helical monomers (3-12), which generates a non aligned supermolecular structure with an aqueous core through which ions can cross lipid membranes. Alamethicin catalyzes the exchange of protons for monovalent cations with little difference in affinities and has the ability to transport cations through biological and artificial lipid membranes. Alamethicin can be used for the permeabilization of mitochondria without affecting the outer or inner membranes.
in vitro
alamethicin was identified as an antibiotic peptide belonging to membrane active peptides of fungal origin with an unusual amphiphilic amino acid, 2-aminoisobutyric acid, which can induce helical peptide structures strongly, leading to the formation of voltage-gated ion channels in the cell membrane bilayers. alamethicin is also widely used as agent to induce various defense responses and physiological in eukaryotic cells [1]. in addition, alamethicin was quite often used to evaluate voltage gating, ion channel assembly, as well as peptide-membrane interactions [2, 3].
Purification Methods
Recrystallise alamethicin from MeOH. [Panday et al. J Am Chem Soc 99 8469 1977.] The acetate has m 195-180o from MeOH/Et2O, and the acetate-methyl ester [64936-53-4] has m 145-140o from aqueous MeOH.
References
1) Woolley?et al. (2007),?Channel-forming activity of alamethicin: effects of covalent tethering; Biodivers.,?4?1323 2) Quist?et al. (1989),?Regulation of polyphosphoinositide synthesis in cardiac membranes; Arch. Biochem. Biophys.,?271?21 3) Nakagawa?et al. (1986),?Adenylate cyclase in sarcoplasmic reticulum of skeletal muscle: distribution orientation and regulation;?J. Cyclic Nuc. Prot. Phosphor. Res.,?11?237 4) Korge?et al. (2016),?Reactive oxygen species production in cardiac mitochondria after complex I inhibition: Modulation by substrate-dependent regulation of the NADH/NAD(+) ratio; Free Radic. Biol. Med.,?96?22
ALAMETHICINRESEARCH GRADE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15956 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| BOC Sciences | +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Zhejiang Hangyu API Co., Ltd | +8617531972939 | anna@api-made.com | China | 2944 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33350 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Nanjing Shizhou Biology Technology Co.,Ltd | 025-85560043 13675144456 | sean.lv@synzest.com | China | 11442 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 94838 | 76 |
View Lastest Price from ALAMETHICINRESEARCH GRADE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-02 | Alamethicin
27061-78-5
|
US $10.00-100.00 / kg | 1kg | 99% | 5000 Metric Ton/Month | Wuhan Xinhao Biotechnology Co., Ltd | |
 |
2019-12-26 | ALAMETHICINRESEARCH GRADE
27061-78-5
|
US $1.00 / g | 1g | ≥98% | g/kg/Ton | Career Henan Chemical Co |
-

- Alamethicin
27061-78-5
- US $10.00-100.00 / kg
- 99%
- Wuhan Xinhao Biotechnology Co., Ltd
-

- ALAMETHICINRESEARCH GRADE
27061-78-5
- US $1.00 / g
- ≥98%
- Career Henan Chemical Co