Empagliflozin
- CAS No.
- 864070-44-0
- Chemical Name:
- Empagliflozin
- Synonyms
- (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-6-(hydroxyMethyl)-tetrahydro-2H-pyran-3,4,5-triol;Empaglifloin;Glyxambi;mpagliflozin;(1S)-1,5-Anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-D-glucitol;ozin;Empag;CS-798;EmpagL;CE0108
- CBNumber:
- CB52627802
- Molecular Formula:
- C23H27ClO7
- Molecular Weight:
- 450.91
- MDL Number:
- MFCD22566222
- MOL File:
- 864070-44-0.mol
| Boiling point | 665℃ |
|---|---|
| Density | 1.398 |
| Flash point | 356℃ |
| storage temp. | 2-8°C |
| solubility | insoluble in H2O; ≥20.75 mg/mL in DMSO; ≥7.06 mg/mL in EtOH with ultrasonic |
| form | solid |
| pka | 13.23±0.70(Predicted) |
| color | White |
| Stability | Hygroscopic |
| InChIKey | OBWASQILIWPZMG-QZMOQZSNSA-N |
| SMILES | C1=C(CC2=CC([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)=CC=C2Cl)C=CC(O[C@H]2CCOC2)=C1 |
| FDA UNII | HDC1R2M35U |
| NCI Drug Dictionary | empagliflozin |
| ATC code | A10BK03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361-H362 |
| Precautionary statements | P201-P260-P263-P264-P270-P308+P313-P201-P202-P281-P308+P313-P405-P501 |
Empagliflozin price More Price(42)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 17375 | Empagliflozin ≥98% | 864070-44-0 | 5mg | $49 | 2024-03-01 | Buy |
| Cayman Chemical | 17375 | Empagliflozin ≥98% | 864070-44-0 | 10mg | $87 | 2024-03-01 | Buy |
| Cayman Chemical | 17375 | Empagliflozin ≥98% | 864070-44-0 | 50mg | $216 | 2024-03-01 | Buy |
| Cayman Chemical | 17375 | Empagliflozin ≥98% | 864070-44-0 | 100mg | $333 | 2024-03-01 | Buy |
| TRC | E521510 | Empagliflozin | 864070-44-0 | 10mg | $80 | 2021-12-16 | Buy |
Empagliflozin Chemical Properties,Uses,Production
Description
Empagliflozin(trade name Jardiance,864070-44-0) is an inhibitor of the sodium glucose co-transporter-2 (SGLT-2), and causes sugar in the blood to be excreted by the kidneys and eliminated in urine. On August 1, 2014, the Food and Drug Administration (FDA) officially approved the drug for the treatment of type 2 diabetes, to improve and control blood glucose of adults. Empagliflozin is the third SGLT-2 inhibiting drugs approved by FDA. Another two SGLT-2 inhibitor drugs, canagliflozin and dapagliflozin, belonging to Johnson Pharmaceuticals, AstraZeneca and Bristol-Myers Squibb respectively, are approved by FDA in November 2013 and January 2014 respectively. The new drug Empagliflozin’s application to FDA can be described as twists and turns. In March 2014, due to a large particle contamination incident at the empagliflozin production plant in Boehringer, the application of new drug submitted by the Boehringer-Eli Lilly Alliance was rejected by FDA. In June 2014, after reviewing the summary and material submitted by Boehring in March, confirming that the quality management and compliance system of the Boehring drug production facility was acceptable, FDA withdrew the previously issued warning letter. The Boehringer-Eli Lilly Alliance also filed an application with the FDA on June 17th.
Clinical research
FDA approval of empagliflozin(Jardiance,864070-44-0) was based on the results of seven clinical trials conducted in nearly 4,500 patients with type 2 diabetes mellitus. Compared to the placebo group, all patients taking empagliflozin(Jardiance) had a significantly lower HbA1c level. The most common adverse reactions are urinary tract infections and female reproductive system infections.
On March 21, 2014, European Medicines Agency (EMA) Commission on Human Medicines recommended the approval of the sodium glucose co-transporter-2 (SGLT2) inhibitor (empagliflozin) for the treatment of adult type 2 diabetes mellitus.
On May 23, 2014, European Medicines Agency (EMA) approved for empagliflozin’s listing in Europe.
On June 16, 2014, the Boehringer-Erya Diabetes Federation released the latest data of empagliflozin Phase II clinical trials at the 74th American Diabetes Association Science Conference (ADA2014). In a two-year study, empagliflozin or glimepiride was used in the treatment of adult patients with type 2 diabetes based on metformin. The results showed that empagliflozin(Jardiance) reduced HbA1c more significantly compared with glimepiride, while the effect of weight and blood pressure treatment are equivalent with glimepiride. Another 52-week study was conducted in patients with type 2 diabetes who were still unable to adequately control blood glucose levels with high doses of insulin (with or without metformin). The results showed that compared with placebo, Jardiance significantly reduced blood glucose levels and weight, and reduced the dose of insulin, with Jardiance or placebo on a daily basis with multiple injections of insulin. In both studies, the safety of empagliflozin was consistent with previous studies.
FDA-related reports write that the drug should not be used in the following types of patients: type 1 diabetes, elevated blood or urine ketones (diabetic ketoacidosis), severe kidney damage, end-stage renal disease or dialysis patients. The most common side effects are urinary tract infections and female genital infections.
Empagliflozin can cause dehydration and lead to decreased blood pressure, resulting in patients with dizziness or fainting and decreased renal function. The risk of older patients, especially those with impaired renal function and diuretics, appears to be greater.
FDA requires Boehring and Eli Lilly to conduct four post-marketing studies: complete ongoing cardiovascular outcome trials, pediatric pharmacokinetics and pharmacodynamics trials, pediatric safety and efficacy trials, toxicity test for the drug (especially effect for renal function, bone and growth and development)
Precautions
Do NOT use empagliflozin if:
- you are allergic to any ingredient in empagliflozin
- you have type 1 diabetes
- you have high blood or urine ketone levels (diabetic ketoacidosis)
- you have severe kidney problems or are on dialysis
References
https://en.wikipedia.org/wiki/Empagliflozin
https://www.drugs.com/cdi/empagliflozin.html
Description
Empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, was originally discovered by Boehringer Ingelheim and codeveloped and co-marketed through research collaboration with Eli Lilly and Co. It was first approved by European Medicine Agency (EMA) in May 2014, followed by the approval of the US FDA in August 2014. SGLT2 inhibitors are anewclass of glucose-lowering agents developed for the treatment of type 2 diabetes mellitus, which have a mechanism of action that is independent of pancreatic b-cell function or the degree of insulin resistance. Consequently, SGLT2 inhibitors have the potential to be of use not only as standalone therapy but also in combination with any of the existing classes of glucose-lowering agents, including insulin. Empagliflozin selectively inhibits SGLT2, which in turn prevents glucose reabsorption by excreting excess glucose in the urine.
Uses
Empagliflozin is a novel, potent and selective SGLT-2 inhibitor, improves glycaemic control and features of metabolic syndrome in diabetic rats.
Definition
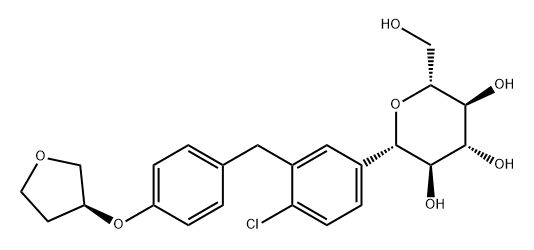
ChEBI: Empagliflozin is a C-glycosyl compound consisting of a beta-glucosyl residue having a (4-chloro-3-{4-[(3S)-tetrahydrofuran-3-yloxy]benzyl}phenyl group at the anomeric centre. A sodium-glucose co-transporter 2 inhibitor used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. It has a role as a sodium-glucose transport protein subtype 2 inhibitor and a hypoglycemic agent. It is a C-glycosyl compound, an aromatic ether, a tetrahydrofuryl ether and a member of monochlorobenzenes.
Clinical Use
Selective and reversible inhibitor of sodium-glucose co-transporter 2:Treatment of type 2 diabetes
Side effects
Empagliflozin had a low incidence of adverse reactions. Compared with the placebo group, the incidence of common adverse reactions was 2%, including urinary tract infection, female reproductive system fungal infection, upper respiratory tract infection, polyuria, dyslipidemia (dose-related low-density lipoprotein increased), arthralgia, fungal infection of male reproductive system, hypoglycemia (the incidence of hypoglycemia increased when empagliflozin was combined with insulin or sulfonylurea hypoglycemic drugs), nausea. Other adverse reactions less than 2% were common in thirst (polydipsia, polydipsia), hypovolemia, and renal dysfunction.
Synthesis
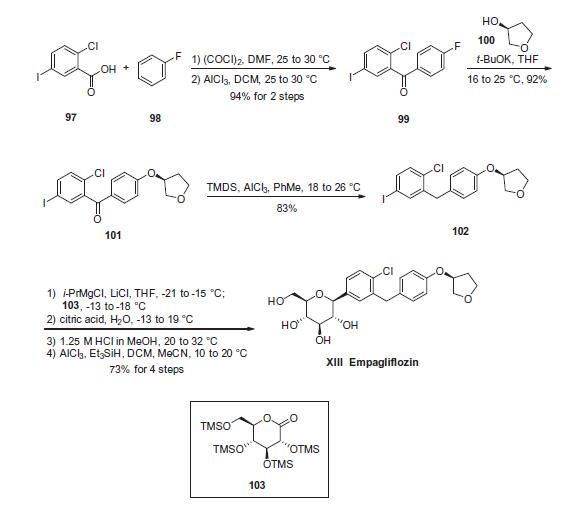
Commercial 5-iodo-2-chlorobenzoic acid (97) was first converted to the corresponding acid chloride, prior to subjection to commercially available fluorobenzene (98) under Friedel¨CCrafts conditions to generate the desired fluorobenzophenone 99 in 94% yield after isolation by recrystallization from aqueous isopropanol. The fluorobenzophenone (99) was then reacted with commercially available (S)-3-hydroxytetrahydrofuran (100) and potassium tertbutoxide in THF to afford ethereal benzophenone 101. Next, removal of the ketone functionality within 101 was achieved through the use of 1,1,3,3-tetramethyldisiloxane (TMDS) in the presence of aluminum chloride in toluene to deliver diaryl iodide 102. This iodide was subsequently converted to the corresponding Grignard reagent and subjected to gluconolactone 103, giving rise to an intermediate lactol which was then sequentially treated with aqueous citric acid, methanolic HCl, and triethylsilyl hydride and aluminum trichloride to ultimately furnish empagliflozin (XIII) in 73% yield across the four-step protocol.
target
SGLT-2
Metabolism
In vitro studies suggested that the main route of metabolism is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases UGT2B7, UGT1A3, UGT1A8, and UGT1A9Following administration of oral [14C]-empagliflozin solution to healthy volunteers, approximately 96% of the drug-related radioactivity was eliminated in faeces (41%) or urine (54%). The majority of drug-related radioactivity recovered in faeces was unchanged parent drug and approximately half of drug-related radioactivity excreted in urine was unchanged parent drug
storage
-20°C
Mode of action
Empagliflozin is an orally available competitive inhibitor of sodium-glucose co-transporter 2 (SGLT2; SLC5A2) with antihyperglycemic activity. Upon oral administration, empagliflozin selectively and potently inhibits SGLT2 in the kidneys, thereby suppressing the reabsorption of glucose in the proximal tubule. Inhibition of SGLT2 increases urinary glucose excretion by the kidneys, resulting in a reduction of plasma glucose levels in an insulin-independent manner. Inhibition of SGLT2 in the kidneys also suppresses the renal reabsorption of 1,5-anhydroglucitol (1,5AG). This lowers serum 1,5AG and neutrophil 1,5-anhydroglucitol-6-phosphate (1,5AG6P) levels, which may improve neutropenia and neutrophil dysfunction in patients with glycogen storage disease type Ib (GSD Ib). SGLT2, a transport protein exclusively expressed in the proximal renal tubules, mediates approximately 90% of renal glucose reabsorption from tubular fluid.
pubchem.ncbi.nlm.nih.gov/compound/Empagliflozin

Empagliflozin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Rochi Pharmaceutical Co.,Ltd. | 21-38751876 +8615000076078 | info@rochipharma.com | China | 431 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 18958018566; | info@afinechem.com | China | 15377 | 58 |
| Senova Technology Co. Ltd. | +86-0755-86703119 +8618503098836 | info@senovatech.com | China | 350 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 | sales001@rokechem.com | China | 255 | 58 |
| Seasons Biotechnology Co., Ltd. | +86-0576-89232655 +86-13566878689 | info@seasonsbio.com | China | 46 | 58 |
| TAIZHOU YUXIN BIOTECHNOLOGY CO,.LTD | +86-576-88902229;+86-0576-88902229 +8613968687450 | yuxin@yuxchem.com | China | 122 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 371 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
Related articles
- Empagliflozin protects the heart against ischemia/reperfusion-induced sudden cardiac death
- Empagliflozin is a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor used to lower blood sugar in adults with type 2 ....
- Jan 5,2024
- An analytical study of empagliflozin in the risk of gout
- Empagliflozin is an FDA-approved antidiabetic drug in the sodium-glucose cotransporter protein (SGLT-2) class of diabetes medi....
- Dec 13,2023
- Pharmacological effects of empagliflozin
- Empagliflozin is a type 2 sodium glucose cotransporter (SGLT-2, sodium-dependent glucose cotransporter2) inhibitor jointly dev....
- Apr 20,2022
View Lastest Price from Empagliflozin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-21 | Empagliflozin
864070-44-0
|
US $0.00-0.00 / kg | 1kg | 99 | 20 tons | Shanghai Affida new material science and technology center | |
 |
2024-05-20 | Empagliflozin
864070-44-0
|
US $0.00 / kg | 1kg | 98% HPLC | 500 kgs | PUSHAN INDUSTRIAL (SHAANXI) CO.,LTD | |
 |
2024-05-17 | Empagliflozin
864070-44-0
|
US $800.00-780.00 / kilograms | 10kilograms | 99% | 100tons | Hebei Dangtong Import and export Co LTD |
-

- Empagliflozin
864070-44-0
- US $0.00-0.00 / kg
- 99
- Shanghai Affida new material science and technology center
-

- Empagliflozin
864070-44-0
- US $0.00 / kg
- 98% HPLC
- PUSHAN INDUSTRIAL (SHAANXI) CO.,LTD
-

- Empagliflozin
864070-44-0
- US $800.00-780.00 / kilograms
- 99%
- Hebei Dangtong Import and export Co LTD
864070-44-0(Empagliflozin)Related Search:
1of4





