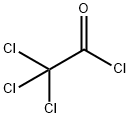
Trichloroacetyl chloride
- CAS No.
- 76-02-8
- Chemical Name:
- Trichloroacetyl chloride
- Synonyms
- 2,2,2-trichloroAcetyl chloride;CCl3COCl;Superpalite;Trichloroacetic chloride;TRICHLOROACETIC ACID CHLORIDE;trichloroacetyl;Green cross gas;Trichloroacetyl chlo;Trichloroacetochloride;trichloro-acetylchlorid
- CBNumber:
- CB6854254
- Molecular Formula:
- C2Cl4O
- Molecular Weight:
- 181.83
- MDL Number:
- MFCD00000792
- MOL File:
- 76-02-8.mol
- MSDS File:
- SDS
| Melting point | -57 °C |
|---|---|
| Boiling point | 114-116 °C(lit.) |
| Density | 1.629 g/mL at 25 °C(lit.) |
| vapor pressure | 16 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 100 °C |
| storage temp. | Refrigerator, Under inert atmosphere |
| solubility | Chloroform (Soluble), Ethyl Acetate (Soluble) |
| form | Oil |
| color | Colourless |
| Water Solubility | reacts violently |
| Sensitive | Moisture Sensitive |
| BRN | 774120 |
| Stability | Stable. Reacts violently with water. Incompatible with alcohols, oxidizing agents, strong bases. |
| CAS DataBase Reference | 76-02-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 9SN86T76Y6 |
| NIST Chemistry Reference | Trichloroacetyl chloride(76-02-8) |
| EPA Substance Registry System | Trichloroacetyl chloride (76-02-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H314-H318-H330 | |||||||||
| Precautionary statements | P303+P361+P353-P304+P340-P305+P351+P338-P320-P405-P501a | |||||||||
| Hazard Codes | T+,T | |||||||||
| Risk Statements | 14-22-26-34-35-29 | |||||||||
| Safety Statements | 26-28-36/37/39-45-8-28A-23 | |||||||||
| RIDADR | UN 2442 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AO7140000 | |||||||||
| F | 19 | |||||||||
| Hazard Note | Very Toxic | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29159080 | |||||||||
| NFPA 704 |
|
Trichloroacetyl chloride price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 151599 | Trichloroacetyl chloride 99% | 76-02-8 | 25G | $39.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 151599 | Trichloroacetyl chloride 99% | 76-02-8 | 100G | $108 | 2024-03-01 | Buy |
| Sigma-Aldrich | 151599 | Trichloroacetyl chloride 99% | 76-02-8 | 500G | $292 | 2024-03-01 | Buy |
| Alfa Aesar | B21675 | Trichloroacetyl chloride 99% | 76-02-8 | 50g | $65.1 | 2024-03-01 | Buy |
| Alfa Aesar | B21675 | Trichloroacetyl chloride 99% | 76-02-8 | 250g | $282 | 2024-03-01 | Buy |
Trichloroacetyl chloride Chemical Properties,Uses,Production
Chemical Properties
Trichloroacetyl chloride is similar to dichloroacetyl chloride. It is a clear colourless liquid. Decomposes in water; soluble in alcohol.The acid chloride can be hydrolyzed at 75 – 85°C with water to form the free acid and hydrolyzed with ammonium hydroxide or concentrated sodium carbonate solution to form the salts.
Uses
Trichloroacetyl Chloride is a reagent used to synthesize pyrazol-furan carboxamide analogues, which can act as Akt kinase inhibitors and pyrrolostatin, which can act as a lipid peroxidation inihibitor (1,2).
Uses
Trichloroacetyl chloride can be used for the manufacture of the esters and the anhydrides of trichloroacetic acid.It was used in the preparation of dihydro-1H-benzindoles. It was also used in the synthesis of 3-alkylbenzoxazolones.
Preparation
The acetyl chloride is prepared from trichloroacetic acid and various inorganic acid chlorides (e.g., SOCl2, PCl3) or with P2O5 and HCl. More useful methods are the oxidation of tetrachloroethylene with fuming sulfuric acid, oxygen, or fuming nitric acid and sulfuric acid at 18-20°C, or from the reaction of pentachloroethane and dry oxygen under UV light. It has been obtained in 37 % yield from carbon tetrachloride and carbon monoxide in the presence of aluminum chloride at 200°C and high pressure.The most common production method is the gas-phase, photochemical oxidation of tetrachloroethylene with oxygen. The reaction is initiated with UV light, with radioactive irradiation, or it is sensitized with chlorine or iodine.
General Description
A colorless volatile liquid with a strong odor. Denser than water. Contact severely irritates skin, eyes and mucous membranes. May be very toxic by ingestion and inhalation. May be combustible.
Reactivity Profile
Trichloroacetyl chloride is incompatible with water, with strong oxidizing agents, alcohols, bases (including amines). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
Highly toxic by ingestion and inhalation; strong irritant to skin and tissues.
Fire Hazard
Material may burn but does not ignite readily. Poisonous if inhaled or swallowed; skin contact poisonous. Contact may cause burns to skin and eyes.
Potential Exposure
Used in chemical syntheses.
Shipping
UN2442 Trichloroacetyl chloride, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, ethers and metal salts. Violent reaction with water, forming hydrochloric acid and trichloroacetic acid.
Waste Disposal
Do not discharge into drains or sewers. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration (oxides of nitrogen are removed from the effluent gas by scrubbers and/or thermal devices) Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Trichloroacetyl chloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12471 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21689 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Changzhou Ansciep Chemical Co., Ltd. | +86 519 86305871 | sales@ansciepchem.com | CHINA | 4242 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Shanghai Worldyang Chemical Co.,Ltd. | ,+86-21-56795779; +8613651600618 | sales@worldyachem.com | China | 879 | 58 |
| Henan Xiangtong Chemical Co., Ltd. | 86-371-61312303 | info@hnxtchem.com | CHINA | 292 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from Trichloroacetyl chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-13 | Trichloroacetyl chloride
76-02-8
|
US $100.00 / drum | 1drum | 88 | 5000 | Hebei Fengqiang Trading Co., LTD | |
 |
2023-08-16 | Trichloroacetyl chloride
76-02-8
|
US $30.00 / KG | 1KG | >99% | 20tons | Weijer International Trade (Hebei) Co., Ltd | |
 |
2023-06-20 | Trichloroacetyl chloride
76-02-8
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- Trichloroacetyl chloride
76-02-8
- US $100.00 / drum
- 88
- Hebei Fengqiang Trading Co., LTD
-

- Trichloroacetyl chloride
76-02-8
- US $30.00 / KG
- >99%
- Weijer International Trade (Hebei) Co., Ltd
-

- Trichloroacetyl chloride
76-02-8
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd