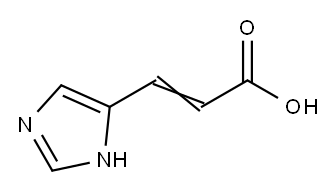
Urocanic acid
- CAS No.
- 104-98-3
- Chemical Name:
- Urocanic acid
- Synonyms
- 4-IMIDAZOLEACRYLIC ACID;(E)-3-(1H-imidazol-5-yl)acrylic acid;3-(1H-IMIDAZOL-4-YL)ACRYLIC ACID;SKL080;UROCANIC ACID;Urocanic caid;Urocalic acid;urocanoic acid;UROCANINIC ACID;Urocanic acid,99%
- CBNumber:
- CB8750635
- Molecular Formula:
- C6H6N2O2
- Molecular Weight:
- 138.12
- MDL Number:
- MFCD00005203
- MOL File:
- 104-98-3.mol
- MSDS File:
- SDS
| Melting point | 226-228 °C(lit.) |
|---|---|
| Boiling point | 253.51°C (rough estimate) |
| Density | 1.3471 (rough estimate) |
| refractive index | 1.5100 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | 1.5g/l |
| pka | 2.94±0.10(Predicted) |
| form | Powder |
| color | White to beige |
| Water Solubility | SLIGHTLY SOLUBLE |
| BRN | 81405 |
| InChI | InChI=1S/C6H6N2O2/c9-6(10)2-1-5-3-7-4-8-5/h1-4H,(H,7,8)(H,9,10) |
| InChIKey | LOIYMIARKYCTBW-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C=CC1NC=NC=1 |
| CAS DataBase Reference | 104-98-3(CAS DataBase Reference) |
| EWG's Food Scores | 1-3 |
| FDA UNII | G8D26XJJ3B |
| EPA Substance Registry System | 2-Propenoic acid, 3-(1H-imidazol-4-yl)- (104-98-3) |
SAFETY
Risk and Safety Statements
| Safety Statements | 22-24/25 |
|---|---|
| WGK Germany | 3 |
| RTECS | NI3425200 |
| HazardClass | IRRITANT |
| HS Code | 29332990 |
Urocanic acid price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 859796 | 4-Imidazoleacrylic acid 99% | 104-98-3 | 5g | $54.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 859796 | 4-Imidazoleacrylic acid 99% | 104-98-3 | 25g | $258 | 2024-03-01 | Buy |
| Sigma-Aldrich | 859796 | 4-Imidazoleacrylic acid 99% | 104-98-3 | 100g | $813 | 2024-03-01 | Buy |
| TRC | U847063 | UrocanicAcid | 104-98-3 | 250mg | $50 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FU29984 | Urocanic acid | 104-98-3 | 5G | $50 | 2021-12-16 | Buy |
Urocanic acid Chemical Properties,Uses,Production
Chemical Properties
White to beige fine powder.
Uses
Urocanic Acid is a biomarker in the fecal metabolic profiling of breast cancer patients.
Definition
ChEBI: Urocanic acid is an alpha,beta-unsaturated monocarboxylic acid that is prop-2-enoic acid substituted by a 1H-imidazol-4-yl group at position 3. It is a metabolite of hidtidine. It has a role as a chromophore and a human metabolite. It is an alpha,beta-unsaturated monocarboxylic acid and a member of imidazoles. It is a conjugate acid of a urocanate.
Biological Functions
Urocanic acid, produced in the upper layers of mammalian skin, is a major absorber of ultraviolet radiation (UVR). Urocanic acid (UCA) is formed in the upper layers of the epidermis where filaggrin, a histidine-rich filamentous protein produced after caspase-14 cleavage of profilaggrin, is broken down by proteinases into component amino acids.
General Description
4-Imidazoleacrylic acid also known as urocanic acid is a natural metabolite derived from histidine. It is majorly used as a UV chromophore with a strong absorption spectrum in the UV-B region in the range of 300-280 nm.
Synthesis
A method of producing trans-urocanic acid, which involves treating D-glucose with aqueous ammonia and formalin in molar ratio D-glucose: aqueous ammonia: formalin equal to 1:24:2.7, in water in the presence of basic copper carbonate at temperature 85-90°C for 3 hours to form (1S,2S,3S)-1-(1H-imidazol-4-yl)-butane-1,2,3,4-tetrazole, which is extracted in form of a hydrochloride. Through periodate splitting in water, the obtained (1S,2S,3S)-1-(1H-imidazol-4-yl)-butane-1,2,3,4-tetrazole is converted at room temperature to 1H-imidazole-4-carbaldehyde, condensation of which, in acetic anhydride in the presence of anhydrous potassium acetate at temperature 120°C for 2 hours, leads to formation of trans-urocanic acid with subsequent extraction thereof from the reaction mass.
Purification Methods
Crystallise the acid from water and dry it at 100o. The trans-isomer [3465-72-3] has m 225o (229-230o, 230-231o or 231o(dec, from H2O) and pK1 3.5 and pK2 5.6, and the picrate has m 225o(dec, from H2O). The cis-isomer [7699-35-6] has m 175-176o (178-179o or 180-184o dec, from H2O) and pK1 3.0 and pK2 6.7, and the picrate has m 204o (from H2O). [Beilstein 25 H 124, 25 I 536, 25 II 121, 25 III/IV 786.]
Urocanic acid Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Unipharm pharmaceutical industry Co., Ltd | 536-8266195 +8613953676784 | lee@unipharm-sd.com | China | 1206 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12455 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
View Lastest Price from Urocanic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-11-13 | Urocanic acid
104-98-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-09-06 | Urocanic acid
104-98-3
|
US $0.00-0.00 / KG | 50KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Urocanic acid
104-98-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Urocanic acid
104-98-3
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
104-98-3(Urocanic acid)Related Search:
1of4