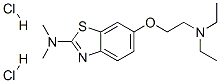Diamthazole Dihydrochloride
- CAS No.
- 136-96-9
- Chemical Name:
- Diamthazole Dihydrochloride
- Synonyms
- D01702;DIMAZOL;Asterol (tn);Dimazole dihydrochloride;Diamthazole dihydrochloride;Dimazole dihydrochloride (jan);6-[2-(diethylamino)ethoxy]-N,N-dimethylbenzothiazol-2-amine dihydrochloride
- CBNumber:
- CB8924304
- Molecular Formula:
- C15H25Cl2N3OS
- Molecular Weight:
- 366.3495
- MDL Number:
- MFCD01663307
- MOL File:
- 136-96-9.mol
| Melting point | 240-243° |
|---|---|
| FDA UNII | 3LF09TBB5W |
Diamthazole Dihydrochloride Chemical Properties,Uses,Production
Originator
Aterola,Roche,US,1951
Definition
ChEBI: Diamthazole dihydrochloride is a member of benzothiazoles.
Manufacturing Process
19.4 g of 2-dimethylamino-6-hydroxybenzothiazole (MP 245°C) were sludged
in a 500 cc three-necked flask with 250 cc of chlorbenzene. Then 4.4 g of
sodium hydroxide flakes were added and the mixture heated with agitation to
90°C. 4 cc of water were dropped in, and the mixture then heated slowly to
the boil while about 500 cc of the water-containing chlorbenzene were distilled
off. 50 cc of dry chlorbenzene were then added and the distillation was
continued until about 30 cc of the chlorbenzene were distilled off. The residue
was the sodium salt of thiazole in chlorbenzene. To the residue were added at
90°C, 15 g of fresh distilled 1-diethylamino-2-chloroethane. The mixture was
then refluxed at 133°C for three hours, then cooled to 35°C. 75 cc of water
and 5 cc of (40% by volume) sodium hydroxide solution were added and the
mixture stirred for one hour. The chlorbenzene layer which contained the
reaction product was separated from the aqueous layer in a separatory funnel.
The chlorbenzene solution was then dried with sodium sulfate for twelve
hours. It was then filtered and HCl gas was passed into the chlorbenzene
solution until saturated, while cooling and stirring. The dihydrochloride
precipitated as a white crystalline, sandy powder. The precipitate was filtered
and washed on the funnel with benzene and finally washed with ether. The
filter cake was dried at 80°C to 90°C. The 2-dimethylamino-6-(βdiethylaminoethoxy)-benzothiazole dihydrochloride thus obtained is a white
crystalline powder, MP 240°C to 243°C. It can be recrystallized from ethanol
and ether, or methanol or acetone.
The free base, which is an oil, can be obtained from the aqueous solution of
the dihydrochloride by adding dilute sodium hydroxide or sodium carbonate
solution. The base is soluble in ether, methanol, ethanol, benzene and the
like, but slightly soluble in water.
brand name
Asterol [as dihydrochloride] [Veterinary] (Hoffmann- LaRoche).
Therapeutic Function
Antifungal
Diamthazole Dihydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
Diamthazole Dihydrochloride Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CHEMICAL LAND21 | -- | sales21@chemicalland21.com | South Korea | 6315 | 74 |
| Supplier | Advantage |
|---|---|
| CHEMICAL LAND21 | 74 |