金 化学特性,用途語,生産方法
定義
本品は、ポリエチレンテレフタレート(PET(*))に金を蒸着して積層した薄膜片である。参照表示名称:PET
解説
Au.原子番号79の元素.周期表11族遷移元素.原子量196.96655(2).質量数197(100%)の安定同位体と,169~205に及ぶ放射性同位体が知られている.元素記号はラテン名aurum(黄色を意味する)の最初の二文字.宇田川榕菴は天保8年(1837年)に出版した「舎密開宗」で,これを音訳して浩律母(アウリュム)としている.金は人類にもっとも古くから利用された金属で,B.C.5000年のエジプトの遺跡からも金の器具が発見されている.世界最大の塊金の発見は,1869年にオーストラリアのビクトリアのもので2520オンス(約71 kg)で,これにより2280オンスの純金が得られた.わが国においては,続日本紀に,聖武天皇の天平21年(749年)に陸奥の国より産出したことが記されている.歴史的には,佐渡,鴻之舞,串木野金山などがよく知られているが,2007年現在,日本で稼働中の金鉱山は1981年に金脈が見つかった菱刈鉱山(鹿児島県)のみ.同鉱山の鉱石の金含有率は非常に高く1 t 中に平均40 g(40 ppm)もあり,世界の平均値の約10倍で年間7~8 t の金を産出している.2005年には,加えて銅,亜鉛,鉛鉱石精錬の副産物として得られる新産金が約150 t,廃パソコン,携帯電話,めっき廃液などのリサイクルによる再生金が30 t あった.金は大部分自然金として存在し,母岩の石英の風化とともに砂金として産出する.自然金は不純物として銀を含んでいる.また,銅鉱,鉛鉱,黄鉄鉱のなかにも含まれている.地殻中の存在度0.003 ppm.世界の推定全埋蔵量90000 t の40% が南アフリカ,ついでオーストラリア7%,中国,ペルーが各5% 弱.鉱石を水銀でアマルガム化して抽出する混コウ(汞)法,シアン化ナトリウムで処理してシアノ錯イオンとして抽出し(青化法),亜鉛粉末を加えて金を析出させる方法(Merrill Crowe法)に加えて,1970年代から青化法のシアノ錯イオンを活性炭に吸着・分離する方法(carbon-in-pulp法)や,さらに溶媒抽出法が有力となっている.精製は電解法による.黄金色の美しい光沢をもつ金属.結晶は面心立方格子.密度19.32 g cm-3(20 ℃).融点1064.43 ℃,沸点2810 ℃.定圧モル熱容量25.38 J K-1 mol-1(25 ℃).線膨張率0.1424×10-4 K-1(0~100 ℃).熱伝導率315 W m-1 K-1(27 ℃).融解熱12.7 kJ mol-1(1063 ℃).蒸発熱310.5 kJ mol-1(2660 ℃).電気抵抗率2.35×10-6 Ω cm(20 ℃).標準電極電位(Au3+/Au)1.52 V.第一イオン化エネルギー889.9 kJ mol-1(9.225 eV).熱の良導体で銀の73%,また電気の良導体でもあり,銀,銅に次ぎ,電気抵抗率は銀の1.48倍である.金属中でもっとも展延性に富む.硬さ2.5~3.化学的には非常に安定である.単独の酸には不溶.王水に溶けてHAuCl4をつくる.高温では酸素,硫黄とは反応しないが,臭素,塩素とは直接化合する.通常の酸化数1~3.純金を24カラットとして50% の金を含む場合は12カラットと表す.国内では,2005年度の最大用途は,電子部品材料で50% 弱,パソコン,携帯電話用ICパッケージ,プリント基板,リードフレーム,ボンディングワイヤ,コネクター,自動車用電装品など.ついで25% 弱が資産用金地金,宝飾品用10%,歯科・医療用の合金5% などであった.[CAS 7440-57-5][別用語参照]金化合物
森北出版「化学辞典(第2版)
主な性質
- 貴金属に属し、黄金色の輝きと稀少性が特徴(装飾品や財宝として魅力)
- 金の延展性はあらゆる金属のなかで最も大きい
- 自然界では、他の元素と化合し難く、化学的な安定性のため、金は単体として存在(美しい輝きを長いあいだ保つことができる)
- 金は一般に、酸やアルカリなどの溶液とは反応しない。(ただ、塩素を発生する王水には溶ける)
- 金は銀、銅に次いで電気伝導性が高い
- 表面に酸化膜が無いので、圧接加工が容易
化粧品の成分用途
表面改質剤、滑沢剤、抗菌剤、抗黴剤、皮膚コンディショニング剤、着色剤
主な用途
- 電子電気: 通信機器(リレーコネクター、ICセラミックパッケージ、リードフレーム、プリント基板、パソコン、携帯電話、ロボット、電装品)
- 歯科医療(義歯)
- 宝飾品(指輪、ネックレス)
- 美術、工芸品(仏像、宗教用具、金杯)
- メダル(記念メダル)
- その他(金箔、陶磁器)
- 私有保有(金地金)
説明
Metallic gold is virtually insoluble, except in aqua regia. Gold exists in three primary forms, elemental, Au (I), and Au (III). As a precious metal, it is resistant to ionization and generally considered biologically benign in its elemental state.

Gold is not permitted for use in foods, drugs or cosmetics as a coloring agent in the U.S. However, surface decoration of foods under conditions which preclude consumption is not considered to be a food use and is, therefore, exempt from regulations concerning food use. The use of gold in the decoration of food is more popular in Europe, where, probably as the result of its limited use, its use has been accepted.
化学的特性
Gold does not have a distinctive odor at room temperature,
but when heated it emits a sweet odor that is detected with
difficulty. Chloroauric acid composed of yellow-orange
crystalline powder has a faint chlorine odor.
物理的性質
Gold is a soft, malleable, ductile, dense metal with a distinctive yellow color. It is almost aheavy as lead, and both can be cut with a knife. One ounce of gold can be beaten and poundedinto a thin sheet that is only a few molecules thick and that will cover over 300 square feetof surface. Although gold is chemically nonreactive, it will react with chlorine and cyanidesolutions and can be dissolved in aqua regia. Its melting point is 1,064.4°C, its boiling pointis 2,808°C, and its density is 19.3 g/cm
3 (as compared to lead’s density of 11.35 g/cm
3).
同位体
There are a total of 54 isotopes of gold, only one of which is stable: Au-197,which accounts for the element’s total natural existence on Earth. The remaining 53 isotopesare radioactive, are artificially produced in nuclear reactors or particle accelerators,and have half-lives ranging from a few microseconds to a few seconds to a few hours toa few days.
名前の由来
The name “gold” is Anglo-Saxon as well as from the Sanskrit word javal.
The symbol Au is from the Latin word aurum, which means “shining dawn.”
天然物の起源
Gold is the 72nd most abundant element and is widely spread around the world, but it is not evenly distributed through the surface of the Earth. It is usually found in a few concentrated regions, sometimes in pure flake and nugget metallic forms. Most of it exists in conjunction with silver ore, quartz (SiO
2), and the ores of tellurium, zinc, and copper. About one milligram of gold exists in every ton of seawater (this is about 10 parts of gold per trillion parts of seawater, which amounts to a total of about 79 million tons of gold in solution). No economical method of extracting gold from seawater has been developed to recover this treasury of the sea.
Free metallic gold is found in veins of rocks and in ores of other metals. Alluvial gold (placer deposits) is found in the sand and in the gravel at the bottom of streams where it has been deposited as a result of the movement of water over eons. Most gold is recovered from quartz veins called loads and from ores that are crushed.
特性
Gold is not only pleasing to look at but also pleasing to touch, which made it a desirablemetal for human decoration in prehistoric days. It is still the preferred metal for jewelry makingtoday.
Gold is classed as a heavy, noble metal located just below copper and silver in group 11 ofthe periodic table. Gold is a good conductor of electricity as well as an excellent heat reflectorof infrared radiation, which makes it an efficient thin coating on glass in skyscrapers to reflectthe heat of sunlight.
The purity of gold is measured in “carats” (one carat is equal to one part in twenty-four). Thepurest gold is rated at 24 carats, but it is much too soft to be used for jewelry. Good jewelry ismade from 18-carat gold that is 18 parts gold and six parts alloy metal. Thus, an 18-carat goldring is about 75% pure gold and contains about 25% of another metal, such as nickel or copper,to make it harder and more durable. Other alloy metals mixed with gold are silver, platinum, andpalladium—all used to increase gold’s strength and reduce its cost. Some less expensive jewelrycontains 14 or 10 carats of gold (14/24 or 10/24) as well as some other alloy metals.
使用
In manufacture of jewelry; in gold plating other metals; as a standard of currency; most frequently alloyed with silver and copper. For use in medicine, see Gold, Radioactive, Colloidal.
Gold has excellent properties as a reflecting mirror. Gold black is utilized as the absorber of light by
depositing on the plane of light incidence of a thermocouple.
定義
A transition metal that occurs native.
It is unreactive and is very ductile and malleable.
Gold is used in jewelry, often alloyed
with copper, and in electronics and
colored glass. Pure gold is 24 carat; 9 carat
indicates that 9 parts in 24 consist of gold.
Symbol: Au; m.p. 1064.43°C; b.p.
2807°C; r.d. 19.320 (20°C); p.n. 79; r.a.m.
196.96654.
反応性
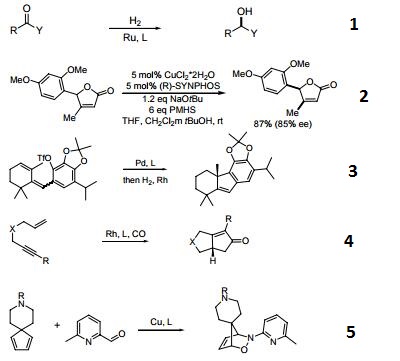
- A chiral diphosphine ligand used in the highly-enantioselective hydrogenation of ketoesters, hydroxyketones, ketophosphonates and succinates.
- A ligand used for the dynamic kinetic resolution of α,β−unsaturated lactones via asymmetric copper-catalyzed conjugate reduction.
- Used in the intramolecular Heck reaction for the synthesis of diterpenoids.
- Used in asymmetric Pauson-Khand reaction.
- Used in asymmetric iminonitroso Diels-Alder reaction.
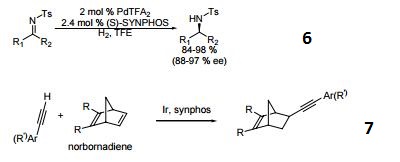
- Palladium catalyzed asymmetric hydrogenation of N-tosyl ketimines.
- Ligand for asymmetric hydroalkynylation of norbornadienes
一般的な説明
Gold nanoparticles PEG 5000 biotin terminated.The preparation of biotinylated gold nanoparticles involves two surface modification steps. First, the carboxyl-terminated alkanethiol is attached to the surface of gold nanoparticles via chemisorption in the presence of a stabilizing agent. This step is followed by the reaction of the carboxyl groups with (+)-biotinyl-3,6,9,-trioxaundecanediamine and 2-(2-aminoethoxy)ethanol.
危険性
Pure gold, if ingested, can cause skin rash or even a sloughing off of skin. It can also causekidney damage and problems with the formation of white blood cells.
応用例(製薬)
Gold has a long-standing tradition in medicine, as it has been used by many nations for thousands of years. From as early as 2500 BC, Arabians, Chinese and Indians used gold compounds for medicinal purposes. In mediaeval times, the elixir aurum potabile , whichwas an alcoholic mixture of herbs with some gold flakes, was sold by medicine men travelling around Europe and this elixir was supposed to cure most diseases. In the nineteenth century, Na[AuCl
4] was reported to treat syphilis, whilst others used it to cure alcoholism. On a more serious note, Koch discovered in 1890 the antibacterial properties of gold cyanide. In vitro experiments with the Mycobacterium tuberculosis showed that gold cyanide has the potential as a tuberculosis therapy. Gold compounds were also investigated for the treatment of RA, when it was believed that RA was caused by bacteria, and many other health problems.
安全性プロファイル
Poison by intravenous
route. Questionable carcinogen with
experimental tumorigenic data by
implantation. Can form explosive
compounds with NH3, NH4OH + aqua regia, H2O2. Incompatible with mixtures
containing chlorides, bromides, or iocbdes (if
they can generate nascent halogens), some
oxidizing materials (especially those
containing halogens), alkali cyanides,
thiocyanate solutions, and double cyanides.
See also GOLD COMPOUNDS.
Structure and conformation
The space lattice of gold belongs to the cubic system, and its face-centered cubic lattice has a lattice
constant of a=0.40705 nm.
金 上流と下流の製品情報
原材料
準備製品