ペメトレキセドニナトリウム
|
- ¥21900 - ¥116700
- 化学名: ペメトレキセドニナトリウム
- 英語名: Pemetrexed Disodium
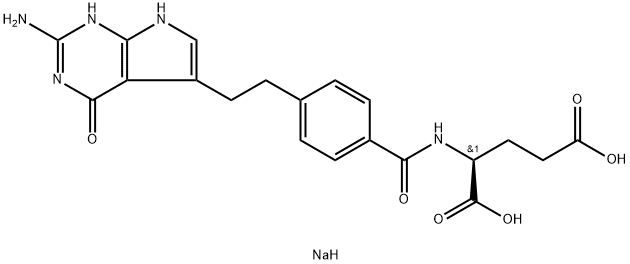
- 別名:ペメトレキセドニナトリウム;ペメトレキセドナトリウム;ロラザール;1,5-二ナトリウム (2S)-2-{[4-(2-{2-アミノ-4-オキソ-1H,4H,7H-ピロロ[2,3-d]ピリミジン-5-イル}エチル)フェニル]ホルムアミド}ペンタンジオアート;N-[4-[2-[(2-アミノ-4-オキソ-4,7-ジヒドロ-3H-ピロロ[2,3-d]ピリミジン)-5-イル]エチル]ベンゾイル]-L-グルタミン酸ジナトリウム;N-[4-[2-(2-アミノ-4-オキソ-4,7-ジヒドロ-1H-ピロロ[2,3-d]ピリミジン-5-イル)エチル]ベンゾイル]-L-グルタミン酸ジナトリウム;アリムタ;チホラール;ペメトレキセドジナトリウム
- CAS番号: 150399-23-8
- 分子式: C20H22N5NaO6
- 分子量: 451.41
- EINECS:604-733-2
- MDL Number:MFCD07779402
3物価
選択条件:
ブランド
- 富士フイルム和光純薬株式会社(wako)
パッケージ
- 100mg
- 250mg
- 1g
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01LKTP1849
- 製品説明ペメトレキセドニナトリウム
- 英語製品説明Pemetrexed Disodium
- 包装単位100mg
- 価格¥21900
- 更新しました2024-03-01
- 購入
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01LKTP1849
- 製品説明ペメトレキセドニナトリウム
- 英語製品説明Pemetrexed Disodium
- 包装単位250mg
- 価格¥39800
- 更新しました2024-03-01
- 購入
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01LKTP1849
- 製品説明ペメトレキセドニナトリウム
- 英語製品説明Pemetrexed Disodium
- 包装単位1g
- 価格¥116700
- 更新しました2024-03-01
- 購入
| 生産者 | 製品番号 | 製品説明 | 包装単位 | 価格 | 更新時間 | 購入 |
|---|---|---|---|---|---|---|
| 富士フイルム和光純薬株式会社(wako) | W01LKTP1849 | ペメトレキセドニナトリウム Pemetrexed Disodium |
100mg | ¥21900 | 2024-03-01 | 購入 |
| 富士フイルム和光純薬株式会社(wako) | W01LKTP1849 | ペメトレキセドニナトリウム Pemetrexed Disodium |
250mg | ¥39800 | 2024-03-01 | 購入 |
| 富士フイルム和光純薬株式会社(wako) | W01LKTP1849 | ペメトレキセドニナトリウム Pemetrexed Disodium |
1g | ¥116700 | 2024-03-01 | 購入 |
プロパティ
融点 :254-258°C (dec.)
貯蔵温度 :Keep in dark place,Inert atmosphere,Store in freezer, under -20°C
溶解性 :Methanol, Water
外見 :Solid
色 :Off-White
安定性: :Hygroscopic
InChIKey :UTEALKYVANXUSG-NVZXTOETNA-N
SMILES :C(C1=CNC2NC(N)=NC(=O)C1=2)CC1C=CC(C(=O)N[C@H](C(=O)O)CCC(=O)O)=CC=1.[NaH] |&1:20,r|
CAS データベース :150399-23-8(CAS DataBase Reference)
貯蔵温度 :Keep in dark place,Inert atmosphere,Store in freezer, under -20°C
溶解性 :Methanol, Water
外見 :Solid
色 :Off-White
安定性: :Hygroscopic
InChIKey :UTEALKYVANXUSG-NVZXTOETNA-N
SMILES :C(C1=CNC2NC(N)=NC(=O)C1=2)CC1C=CC(C(=O)N[C@H](C(=O)O)CCC(=O)O)=CC=1.[NaH] |&1:20,r|
CAS データベース :150399-23-8(CAS DataBase Reference)
安全情報
| 絵表示(GHS): |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注意喚起語: | Warning | |||||||||||||||||||||||||||||||||||
| 危険有害性情報: |
|
|||||||||||||||||||||||||||||||||||
| 注意書き: |
|
説明
Pemetrexed, a pyrrolo[2,3-d]pyrimidine-based antifolate that disrupts cell replication by inhibiting multiple folate-dependent metabolic processes, was initially developed and launched in the US for the treatment of malignant pleural mesothelioma in conjunction with cisplatin. Patients who are not candidates for surgery may benefit from this combination therapy. Clinical data demonstrated that the median overall survival time increased to 12.1 months, compared with 9.3 months for patients receiving cisplatin alone. In August of 2004, the FDA also approved pemetrexed as a second-line treatment of non-small-cell lung cancer (NSCLC). While median survival is comparable to the standard second-line treatment docetaxel, the improved toxicity profile (significant reduction in neutropenia) accelerated the approval for NSCLC. Its effectiveness as an anticancer drug is derived from its ability to gain internal cell access via the reduced folate carrier and membrane folate binding protein transport systems. Once inside, pemetrexed undergoes polyglutamation, and the resultant polyglutamate forms (predominantly the pentaglutamate) inhibit the folate-dependent enzymes thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT). Against recombinant human TS, pemetrexed has a Ki of 109nM while the triglutamate and pentaglutamate forms have Ki values of 1.6nM and 1.3 nM, respectively. All forms of pemetrexed display similar potency against recombinant human DHFR (7 nM), but the pentaglutamate form is significantly more potent against recombinant murine GARFT than the parent (Ki=65nM versus 9.3μM). The selectivity of pemetrexed may be explained by the fact that polyglutamation is more likely to occur in cancer cells compared to normal cells while its prolonged duration of action may be attributed to decreased cellular efflux of the polyglutamate forms. While several different routes have provided pemetrexed, one of the most efficient exploits the propensity of 2,6-diamino-3Hpyrimidin- 4-one to undergo Michael additions at its unsubstituted C-5 position. Using ethyl 4-(4-nitrobut-3-enyl)benzoate as the Michael acceptor, the resulting adduct is then converted to the ultimate precursor for glutamyl coupling via a onepot, three-step process (Nef reaction to transform the nitro to the aldehyde, intramolecular condensation to afford the pyrrole, and saponification of the ethyl ester). A typical treatment regimen involves intravenous administration of pemetrexed, infused over ten minutes, at a dose of 500mg/m2 followed by a thirty minute wash-out period and then cisplatin intravenously over two hours at a dose of 75mg/m2. Both drugs are given on Day 1 of a 21-day cycle. In order to reduce treatment-related hematological and GI toxicity, patients are instructed to take folic acid and vitamin B12 as a prophylactic measure. Pretreatment with a corticosteroid is also recommended to prevent possible skin rashes. Pemetrexed is primarily excreted intact in the urine, with 70–90% of the dose being recovered within 24 hours of administration. The half-life of pemetrexed is 3.5 hours in patients with normal renal function, and the total systemic clearance is 91.8mL/min. As expected, clearance decreases as renal impairment increases. The drug’s plasma protein binding is 81%, and it has a steady state volume of distribution of 16.1 L. The pharmacokinetics of pemetrexed is linear with dose and remains unchanged over multiple treatment cycles. While in vitro studies suggest that pemetrexed would not interfere with drugs metabolized by CYP3A4, CYP2D6, CYP2C9, and CYP1A2, ibuprofen (400mg q.d.) does reduce pemetrexed clearance by 20%. Caution should, therefore, be taken when administering pemetrexed concurrently with ibuprofen to patients with renal insufficiency and should not be given at all to patients whose creatinine clearance is <45mL/min. .関連商品価格
- トリネキサパックエチル
¥12000-584800 - 4-ヒドロキシ安息香酸エチル
¥1100-125000 - 1-メチル-2-ピロリドン
¥1200-254400




