| Company Name: |
Hubei wei shi reagent group ltd., company
|
| Tel: |
027-027-59102966 18717199209 |
| Email: |
2853877583@QQ.com |
| Products Intro: |
Cas:9015-82-1
ProductName:Angiotensin Converting Enzyme
Purity: 95-99%HPLC | Package: 1kg;100g;1g
|
| Company Name: |
Shenzhen Regent Biochemical Technology Co., Ltd.
|
| Tel: |
0755-0755-85201366 18938635012 |
| Email: |
sales@regentsciences.com |
| Products Intro: |
Cas:9015-82-1
ProductName:Angiotensin-converting enzyme
Purity: >=2.0units/mg protein | Package: 1KU;5KU;100KU
|
| Company Name: |
Beijing Boorsen Biotechnology Co., Ltd
|
| Tel: |
400-901-9800 18611424007 |
| Email: |
cis@bioss.com.cn |
| Products Intro: |
Cas:9015-82-1
ProductName:Angiotensin Converting Enzyme from rabbit lung
Package: 0.1UN
|
| Company Name: |
Foshan Treasure Biotechnology Co., Ltd
|
| Tel: |
0757-85921206 18520245316 |
| Email: |
2329783215@qq.com |
| Products Intro: |
Cas:9015-82-1
ProductName:Angiotensin Converting Enzyme
Purity: 99% HPLC | Package: 20mg;50mg;100mg;1g
|
Angiotensin Converting Enzyme manufacturers
- Enzyme
-

- $0.00 / 25KG
-
2023-09-08
- CAS:9001-57-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Enzyme
-

- $100.00 / 1bag
-
2022-06-21
- CAS:556743-18-1
- Min. Order: 1bag
- Purity: 99
- Supply Ability: 500
- Angiotensin
-

- $1.00 / 1g
-
2021-07-20
- CAS:1407-47-2
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 50tons
|
| Product Name: | Angiotensin Converting Enzyme | | Synonyms: | PEPTIDYLDIPEPTIDE HYDROLASE;ANGIOTENSIN CONVERTING ENZYME FROMRABBIT LUNG;ANGIOTENSIN-CONVERTIN-GAMMA-ENZYME;ANGIOTENSIN CONVERTING ENZYME (HUMAN);ACE (HUMAN);ACE (enzyme);Angiotensin converting enzyme 1;Angiotensin I-converting enzyme | | CAS: | 9015-82-1 | | MF: | C2H5NO | | MW: | 59.0672 | | EINECS: | | | Product Categories: | | | Mol File: | 9015-82-1.mol | 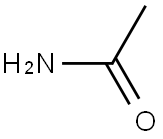 |
| | Angiotensin Converting Enzyme Chemical Properties |
| storage temp. | -20°C | | form | lyophilized powder |
| | Angiotensin Converting Enzyme Usage And Synthesis |
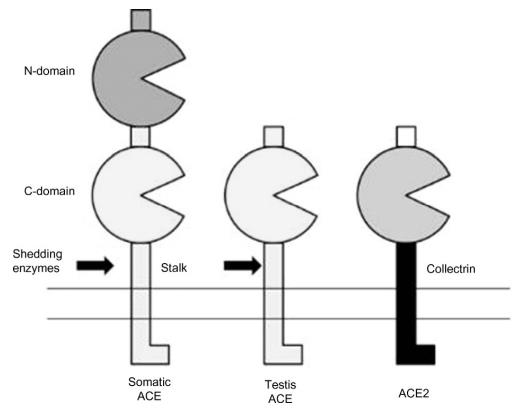
| Structure | Two isozymes of ACE are present in mammals:
somatic ACE and testis ACE. Somatic ACE possesses two
catalytic domains (N- and C-domains) and a C-terminal
transmembrane segment (stalk). Both catalytic
domains are zinc-metallopeptidase with the active motif
HEMGH where the two histidine residues coordinate the
zinc ion. The Km for Hip-His-Leu is 2.51mM. The stalk
anchors the enzyme on the membrane and is suspectible
to be cleaved by shedding enzymes, resulting in plasma
ACE activity. ACE2 is a chimera protein with
a single catalytic domain of ACE, and a C-terminal that
highly resembles collectrin, which may act as a chaperone
protein to deliver other proteins to the brush border
membrane. Somatic and testis ACEs in humans contain 1306 and
665 aa residues, respectively. The testis ACE
only possesses one catalytic domain.
 | | Gene, mRNA, and mRNA | The ACE and ACE2 genes are located at chromosomes
17q23 and Xp22 in humans, respectively. The testis ACE
is transcribed from the same gene with an alternative
transcription starting site on the 13th intron of ACE,
resulting in only a C-domain and a stalk segment with
a unique additional 67 aa N-terminal sequence in
humans. The two catalytic domains are the result of
gene/domain duplication. The duplication occurred
multiple times in evolution as the cnidarians, crustaceans,
insects, and vertebrates possess ACE-like enzymes with
one or two catalytic domains. No expression studies so
far have been performed for nonmammalian ACE and
ACE2. | | Synthesis and release | The expression of ACE is affected by steroids and the
thyroid hormone, but the details of the regulation are not
clear. ACE is under promoter regulation by hypoxiainducing factor 1α (HIF-1α), which upregulates the ACE
expression under hypoxic conditions, resulting in an
increase in Ang II concentration. Under hypoxia, ACE2
will be downregulated; it was shown that it is indirectly
controlled by Ang II, but not HIF-1α. Testis ACE expression control is highly specific and regulated by a
tissue-specific promoter located immediately -59 bp of
the transcription start site, which is frequently used in
testis-specific overexpression studies. Hypoxia induced
by high temperature decreased the gill ACE activity but had no effect on the kidney in the carp. Promoters of
ACE2 from mammals, amphibians, and teleosts drive specific expression in the heart. Cis-Element search results
discovered WGATAR motifs in all putative ACE2 promoters from different vertebrates, suggesting a possible role
of GATA family transcriptional factors in ACE2 expression
regulation. | | Inhibitors | The first ACE inhibitor was a peptide antagonist
called SQ 20,881 (GWPRPEIPP); it was discovered from
snake venom but was not orally active. The snake
venom peptides were further studied to produce the
first orally active form, captopril, which lowers the
blood pressure of essential hypertensive patients. The
most common side effects of captopril are a cough, skin
rash, and loss of taste. Therefore, derivatives such as
enalapril, lisinopril, and ramipril were developed with
fewer side effects. After the discovery of the N- and
C-domains of ACE, specific domain inhibitors were
developed to increase specificity. Ang I is mainly
hydrolyzed by the C-domain in vivo, but BK is hydrolyzed by both domains. Developing a C-domain selective inhibitor (RXPA380) would permit some
degradation of BK by the N-domain; this degradation
could be enough to prevent the accumulation of excess
BK causing angioedema. | | Biological functions | The well-known function of ACE is the conversion of
Ang I to Ang II and the degradation of BK, which plays an
important role in controlling the blood pressure. ACE
also acts on other natural substrates, including encephalin, neurotensin, and substance P. Besides being
involved in blood pressure control, ACE possesses widespread functions including renal development, male
fertility, hematopoiesis, erythropoiesis, myelopoiesis,
and immune responses. ACE2 can convert Ang II to Ang1–7, thereby reducing
the concentration of Ang II and increasing that of Ang1–7.
ACE2 can also convert Ang I to Ang1–9, which is subsequently converted into Ang1–7 by ACE. The high expression of ACE2 favors the balance of Ang1–7 over Ang II,
which accounts for the cardioprotective role of ACE2
via the Ang1–7/Mas signaling pathway. | | Clinical implications | The inclusion (II) or deletion (DD) of the 287 bp Alu
repeat in the 16th intron affects the human plasma ACE
levels. The DD genotype is more frequently found in
patients with myocardial infarction but no convincing
evidence is available on the association of the DD genotype with hypertension. ACE2 was identified as the
receptor for the SARS (severe acute respiratory syndrome) coronavirus. The SARS virus binding downregulates the cellular expression of ACE2, and the binding
induces the clathrin-dependent internalization of the
virus/receptor (SARS/ACE2) complex. Not only has
ACE2 facilitated the invasion of the SARS virus for rapid
replication, but also ACE2 is depleted from the cell membrane. Therefore, the damaging effects of Ang II are
enhanced, resulting in the acute deterioration of lung
tissues. | | Description | ACE possesses dual actions to convert Ang I to Ang II,
and degrade bradykinin. The development of an ACE inhibitor was the first effective drug for hypertension caused by
high renin activity. ACE2 was identified as the receptor
for the SARS (severe acute respiratory syndrome) coronavirus, which caused an epidemic in 2002–2003. ACE was discovered in the mid-1950s through the
observation that the dialysis of plasma and kidney extract
with water and saline before incubation produced two separate pressor substances, Ang I and Ang II, respectively. It
was discovered for a second time in 1966 during the characterization of a bradykinin (BK)-degrading enzyme from
the kidney. This was named kininase II, which later was
found to be the same enzyme as ACE. ACE2 was discovered in 2000 when two independent research groups
cloned homologous ACE that could convert Ang I to
Ang1–9 and yet also be captopril-insensitive. | | Uses | Angiotensin converting enzyme from rabbit lung has been used:
- for measuring inhibitory effect of egg white protein hydrolysates on ACE activity by high performance liquid chromatography (HPLC)
- to measure the ACE inhibition by litchi pericarp and cooked chicken breast using hippuryl-L-histidyl-L-leucine (HHL) as substrate by reverse phase-HPLC (RP-HPLC)3 and HPLC respectively
- in releasing GPI anchored protein in vitro in few cell lines like HeLa, HEK293 and in vivo in mice sperm.
| | General Description | The angiotensin-converting enzyme (ACE) is a dipeptidyl-carboxypeptidase which exists in somatic and testicular isoforms with zinc binding motif HEXXH in their active site. ACE regulates blood pressure through renin-angiotensin system. ACE elevates blood pressure by converting angiotensin I to a key vasoconstrictor angiotensin II and inhibiting a potent vasodilator bradykinin. Inhibition of ACE is a targeted therapeutic strategy for high blood pressure. Several ACE synthetic inhibitory peptides available for clinical use include captopril, enalapril and lisinopril. Currently, developing inhibitory peptides from natural food sources, or phenolic compounds from plant sources to inhibit ACE is underway. ACE plays a critical role in fertilization by releasing the proteins anchored to glycosylphosphatidylinositol (GPI) in sperm membrane. | | Biochem/physiol Actions | Removes C-terminal dipeptides from susceptible substrates, e.g., angiotensin I and bradykinin. | | Clinical Use | ACE has been the target of hypertension control since
the 1970s. ACE inhibitors are prescribed as the sole or
combinational treatment for high blood pressure, for its
dual effects of lowering Ang II and slowing down BK
degradation. In human hypertensive patients, ACE2
levels are lower in both the kidney and heart compared
to normotensive volunteers. | | Purification Methods | Purify ACE by fractionation on DEAE-cellulose, Ca phosphate gel chromatography, elution from Sephadex G-200 and lectin affinity chromatography. The MW varied with glycosidation and is higher by gel filtration. It contains one atom of Zn/mol and has Km values for hydrolysis of hippurylhistidinylleucine and angiotensin I of 2.3 and 0.07 mM, and turnover of 15,430 and 792 mol/min/mol at 37o, respectively. The activity is inhibited by EDTA and increased amounts of Ca ions but required Ca ions. [Das & Soffer J Biol Chem 250 6762 1975, Reviewed by Ehlers & Riordan Biochemistry 28 5311 1989.] |
| | Angiotensin Converting Enzyme Preparation Products And Raw materials |
|