|
Cevane-3,4,14,15,16,20-hexol, 4,9-epoxy-, 3-acetate, (3beta,4alpha,15a lpha,16beta)- Suppliers list
|
|
| | Cevane-3,4,14,15,16,20-hexol, 4,9-epoxy-, 3-acetate, (3beta,4alpha,15a lpha,16beta)- Basic information |
| Product Name: | Cevane-3,4,14,15,16,20-hexol, 4,9-epoxy-, 3-acetate, (3beta,4alpha,15a lpha,16beta)- | | Synonyms: | Cevane-3,4,14,15,16,20-hexol, 4,9-epoxy-, 3-acetate, (3beta,4alpha,15a lpha,16beta)-;4α,9-Epoxycevane-3β,4,14,15α,16β,20-hexol 3-acetate;Zygacine;Cevane-3,4,14,15,16,20-hexol, 4,9-epoxy-, 3-acetate, (3β,4α,15α,16β)- | | CAS: | 2777-79-9 | | MF: | C29H45NO8 | | MW: | 535.68 | | EINECS: | | | Product Categories: | | | Mol File: | 2777-79-9.mol | 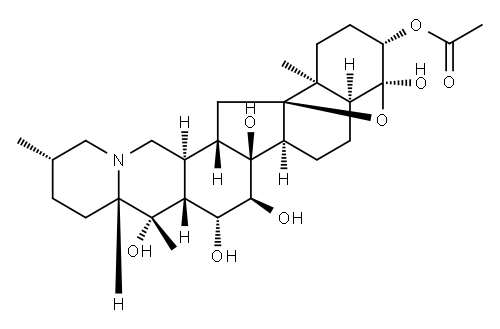 |
| | Cevane-3,4,14,15,16,20-hexol, 4,9-epoxy-, 3-acetate, (3beta,4alpha,15a lpha,16beta)- Chemical Properties |
| Boiling point | 655.9±55.0 °C(Predicted) | | density | 1.40±0.1 g/cm3(Predicted) | | pka | 11.93±0.70(Predicted) |
| | Cevane-3,4,14,15,16,20-hexol, 4,9-epoxy-, 3-acetate, (3beta,4alpha,15a lpha,16beta)- Usage And Synthesis |
| Description | An amorphous, steroidal alkaloid isolated from Zygadenus venenosum, this
base has [α]23/sup>D - 22° (c 1.53, CHC13). The acetonide forms colourless needles from aqueous Me2CO, m.p. 253-5°C (dec.); [α]23/sup>D + 2° (c 1.41, CHCl3), giving a crystalline hydriodide as colourless plates from Me2CO, m.p. 270-1 ° C(dec. ). | | References | Kupchan, Lavie, Zenis.,J. Amer. Chem. Soc., 77,689 (1953)
Gilbertson., Phytochem., 12,2079 (1973) |
| | Cevane-3,4,14,15,16,20-hexol, 4,9-epoxy-, 3-acetate, (3beta,4alpha,15a lpha,16beta)- Preparation Products And Raw materials |
|