- Naratriptan
-

- $16.00 / 1KG
-
2024-01-18
- CAS:121679-13-8
- Min. Order: 1KG
- Purity: 99.90%
- Supply Ability: 20T
- Naratriptan
-

- $15.00 / 1KG
-
2021-07-13
- CAS:121679-13-8
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Naratriptan
-

- $15.00 / 1KG
-
2021-07-09
- CAS:121679-13-8
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Naratriptan Basic information |
| Product Name: | Naratriptan | | Synonyms: | Naratriptan;Naratriptane;N-methyl-2-[3-(1-methyl-4-piperidyl)-1H-indol-5-yl]ethanesulfonamide;Naratriptan(Amerge);N-Methyl-2-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]ethanesulfonamide;Naratriptan1;AMERGE; NARAMIG; GR-85548A;N-methyl-3-(1-methyl-4-piperidinyl)-1H-Indole-5-ethanesulfonamide | | CAS: | 121679-13-8 | | MF: | C17H25N3O2S | | MW: | 335.46 | | EINECS: | | | Product Categories: | API;Indoline & Oxindole | | Mol File: | 121679-13-8.mol | 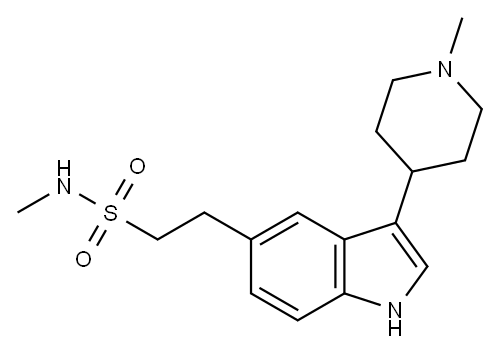 |
| | Naratriptan Chemical Properties |
| Melting point | 170-171° | | Boiling point | 541.3±60.0 °C(Predicted) | | density | 1.227±0.06 g/cm3(Predicted) | | pka | 11.52±0.40(Predicted) |
| | Naratriptan Usage And Synthesis |
| Originator | Amerge,Glaxo Wellcome,UK | | Uses | Antimigraine. | | Uses | Naratriptan is a triptan drug that is used for the treatment of migraine headaches. | | Definition | ChEBI: Naratriptan is a sulfonamide, a member of tryptamines and a heteroarylpiperidine. It has a role as a serotonergic agonist and a vasoconstrictor agent. | | Manufacturing Process | N-Methyl-3-(1,2,3,6-tetrahydro-1-methyl-4-pyridinyl)-1H-indole-5-
ethanesulphonamide oxalate
A solution of N-methyl-1H-indole-5-ethanesulphonamide (1.0 g) in methanol
(50 ml) containing potassium hydroxide (5.6 g) and N-methyl-4-piperidone
(1.0 ml) was heated at reflux for 24 h, cooled, and the resulting solid filtered
off (1.0 g). A sample of the solid (0.2 g) was dissolved in a hot methanolic
solution of oxalic acid (0.06 g), the solution cooled, and the salt precipitated
by adding ethyl acetate (20 ml) and dry ether (50 ml). The salt was filtered
off, and dried in vacuo to give the title compound as a solid (0.12 g), m.p.
87°-90°C (shrinks).
Analysis Found: C,52.2; H,5.6; N,9.5. C17H23N3O2S · C2H2O4 · 0.6H2O
requires C,52.5; H,6.0; N,9.7%.
N-Methyl-3-(1-methyl-4 -piperidinyl)-1H-indole-5-ethansulphonamide
N-Methyl-3-(1,2,3,6-tetrahydro-1-methyl-4-pyridinyl)-1H-indole-5-
ethanesulphonamide oxalate (as the free base) (0.36 g, 0.001 mol) in
absolute alcohol (70 ml) and anhydrous dimethylformamide (5 ml) was
hydrogenated, in the presence of 5% palladium on activated carbon (0.36 g)
at ambient temperature and atmospheric pressure. After 20 h, hydrogen
absorption (25 cm3, theoretical = 24 cm3) ceased. The catalyst was filtered
off and the solvent removed in vacuo to given an opaque gum which solidified
as a soft white solid (0.3 g). Purification by flash chromatography (Sorbsil C60
silica gel, CH2Cl2/EtOH/0.88 ammonia; 50:80:1) gave a colorless oil (0.21 g)
that was triturated with ether to give the title compound (0.17 g) m.p. 156°-
158°C. TLC SiO2(CH2Cl2/EtOH/0.88 ammonia; 50:8:1) Rf 0.4.
N-Methyl-3-(1-methyl-4-piperidinyl)-1H-indole-5-ethanesulphonamide may be
prepared the another way.
A solution of 4-hydrazino-N-methyl-benzenethanesulphonamide (0.5 g) and 1-
methyl-4-piperidineacetaldehyde (0.35 g) in a mixture of water (10 ml) of 2 N
hydrochloric acid (1.0 ml, 2.00 mmol) was stirred for 2 days at room
temperature. A further quantity of the aldehyde (0.35 g) was added and
stirring continued for a further 30 min. The solution was then basified with
8% sodium bicarbonate to pH 8 and extracted with chloroform (3 times 50
ml). The combined organic extracts were dried (Na2SO4) and evaporated in
vacuo to give the crude hydrazone as an oil (1.0 g). A solution of the
hydrazone (1.0 g) in chloroform (20 ml) containing polyphosphate ester (10
g) was heated at reflux for 8 min. The solution was poured onto ice (200 g),
stirred for 2 h treated with 2 M sodium carbonate (20 ml) and extracted with
chloroform (3 times 50 ml). The combined organic extracts were dried
(Na2SO4), evaporated in vacuo and the residue purified by flash
chromatography (silica 9385, 100 g) eluting with CH2Cl2/EtOH/NH3(75:8:1) to
give impure material as a yellow oil. Further flash chromatography (silica
9385, 100 g) eluting with CH2Cl2/EtOH/NH3 (100:8:1) gave the product as an
oil (0.05 g). This was crystallised from ethyl acetate to give the title compound
solid m.p. 156°-157°C. TLC SiO2(CH2Cl2/EtOH/NH3(50:8:1)) Rf 0.6. | | Brand name | Amerge (GlaxoSmithKline). | | Therapeutic Function | Serotonin antagonist, Migraine therapy | | General Description | Naratriptan, the third triptan approved in 1998, is one of themost lipophilic triptans marketed to date. It has a much improvedbioavailability (63% in men and 74% in women), agreater affinity for 5-HT1B/1D receptors (3–6 times), and alower recurrence rate than sumatriptan because of its muchlonger elimination half-life. Naratritan also has a favorableCNS side effect profile when compared with sumatriptanor zolmitriptan because of its metabolic stability,thereby lacking a N-demethylated active metabolite and asignificant renal excretion ( 70% of naratriptan is excretedunchanged and the rest of the administered dose is degradedvia several CYP isozymes). | | Clinical Use | 5HT1
receptor agonist:
Acute treatment of migraine | | Drug interactions | Potentially hazardous interactions with other drugs
Antidepressants: increased CNS toxicity with
citalopram - avoid; possibly increased serotonergic
effects with duloxetine, SSRIs and venlafaxine;
increased serotonergic effects with St John’s wort -
avoid.
Dapoxetine: possible increased risk of serotonergic
effects - avoid for 2 weeks after stopping 5HT1
agonists.
Ergot alkaloids: increased risk of vasospasm - avoid. | | Metabolism | Naratriptan undergoes some hepatic metabolism via
a wide range of cytochrome P450 isoenzymes to form
inactive metabolites.
Naratriptan is excreted by glomerular filtration and active
secretion into the renal tubules. It is mainly excreted
in the urine with 50% of a dose being recovered as
unchanged drug and 30% as inactive metabolites. |
| | Naratriptan Preparation Products And Raw materials |
|