| Company Name: |
Sciencelab.com, Inc.
|
| Tel: |
011.281.354.6400 |
| Email: |
accounting@sciencelab.com |
| Products Intro: |
|
|
| Product Name: | technetium | | Synonyms: | technetium;technetium atom;technetium ISO 9001:2015 REACH | | CAS: | 7440-26-8 | | MF: | Tc | | MW: | 98.906265 | | EINECS: | 231-136-0 | | Product Categories: | | | Mol File: | 7440-26-8.mol |  |
| | technetium Chemical Properties |
| Melting point | 2250 ±50° | | Boiling point | 4265°C (estimate) | | density | 11.000 | | form | hexagonal crystals | | color | hexagonal, hexane crystals, crystalline | | EPA Substance Registry System | Technetium (7440-26-8) |
| | technetium Usage And Synthesis |
| Description | Element with atomic number 43, group VIIB of the periodic table, aw 98.9062, valences of 4, 5, 6, 7; three radioactive isotopes of half-life more than 105 years, also several of relatively short half-life, some of which are β emitters. Technetium was first obtained by the deuteron bombardment of molybdenum, but since has been found in the fission products of uranium and plutonium. The chemistry of technetium has been studied by tracer techniques and is similar to that of rhenium and manganese. The free metal is obtained from reactor fission products by solvent extraction followed by crystallization as ammonium pertechnetate, which is reduced with hydrogen. The metal is silver-gray in appearance, mp 2200C (4000F), d 11.5, slightly magnetic. Compounds of the types TcO2, Tc2O7, NH4TcO4, etc. have been prepared. The pertechnetate ion has strong anticorrosive properties. Technetium and its alloys are superconductors and can be used to create high-strength magnetic fields at low temperature. Tc-99 (metastable) is the mostwidely used isotope in nuclear medicine.
| | Uses | Metallurgical tracer, cryochemistry, corrosion
resistance, nuclear medicine. | | Chemical Properties | closed-packed hexagonal, a=0.2741 nm, c=0.4399nm; enthalpy of sublimation 650 kJ/mol; enthalpy of vaporization ~577 kJ/mol; enthalpy of fusion 33.29 kJ/mol; slowly tarnishes in moist air; when obtained from H2 reduction of ammonium pertechnate, has silvery gray color, and a spongy mass; resembles rhenium in chemical behavior; Debye constant 455K; used as a metallurgical tracer, in nuclear medicine, and to protect against corrosion [HAW93] [MER06] [RAR83] [CRC10] | | Physical properties | As the central member of the triad of metals in group 7, technetium (period 5) has similarphysical and chemical properties as its partners manganese (period 4) above it and rhenium(period 6) below it. The sizes of their atomic radii do not vary greatly: Mn = 127, Tc = 136,and Re = 137. Neither does their level of electronegativity vary significantly: Mn = 1.5, Tc =1.9, and Re = 1.9.
Technetium metal is grayish-silver and looks much like platinum. As with most transitionelements, technetium in pure form is a noncorrosive metal. It requires only 55 ppm of technetiumadded to iron to transform the iron into a noncorroding alloy. Because of technetium’sradioactivity, its use as an alloy metal for iron is limited so as to not expose humans to unnecessaryradiation.
Technetium’s melting point is 2,172°C, its boiling point is 4,877°C, and its density is11.50 g/cm3 . | | Isotopes | There are 47 isotopes. None are stable and all are radioactive. Most are producedartificially in cyclotrons (particle accelerators) and nuclear reactors. The atomicmass of its isotopes ranges from Tc-85 to Tc-118. Most of technetium’s radioactiveisotopes have very short half-lives. The two natural radioisotopes with the longest halflives—Tc-98 = 4.2×10+6 years and Tc-99 = 2.111×10+5 years—are used to establishtechnetium’s atomic weight. | | Origin of Name | Technetium’s name was derived from the Greek word technetos,
meaning “artificial.” | | Occurrence | Technetium is the 76th most abundant element, but it is so rare that it is not found as astable element on Earth. All of it is artificially produced. Even though natural technetium isso scarce that it is considered not to exist on Earth, it has been identified in the light spectrumfrom stars. Using a spectroscope that produces unique lines for each element, scientists areable to view several types of stars. The resulting spectrographs indicate that technetium existsin the stars and thus the universe, but not on Earth as a stable element.
It was the first new element to be produced artificially from another element experimentallyin a laboratory. Today, all technetium is produced mostly in the nuclear reactors of electricalgeneration power plants. Molybdenum-98 is bombarded with neutrons, which then becomesmolybdenum-99 when it captures a neutron. Since Mo-99 has a short half-life of about 66hours, it decays into Tc-99 by beta decay. | | History | Element 43 was predicted on the basis of the
periodic table, and was erroneously reported as having been
discovered in 1925, at which time it was named masurium.
Technetium was actually discovered by Perrier and Segre in
Italy in 1937. It was found in a sample of molybdenum that
was bombarded by deuterons in the Berkeley cyclotron, and
which E. Lawrence sent to these investigators. Technetium
was the first element to be produced artificially. Since its discovery,
searches for the element in terrestrial materials have
been made without success. If it does exist, the concentration
must be very small. Technetium has been found in the
spectrum of S-, M-, and N-type stars, and its presence in
stellar matter is leading to new theories of the production of
heavy elements in the stars. Forty-three isotopes and isomers
of technetium, with mass numbers ranging from 86 to 113,
are known. 97Tc has a half-life of 2.6 × 106 years. Tc has a
half-life of 4.2 × 106 years. The isomeric isotope 95mTc, with
a half-life of 61 days, is useful for tracer work, as it produces
energetic gamma rays. Technetium metal has been produced
in kilogram quantities. The metal was first prepared by passing
hydrogen gas at 1100°C over Tc2S7. It is now conveniently
prepared by the reduction of ammonium pertechnetate with
hydrogen. Technetium is a silvery-gray metal that tarnishes
slowly in moist air. Until 1960, technetium was available only
in small amounts and the price was as high as $2800/g, but
the price is now of the order of $100/g. The chemistry of technetium
is similar to that of rhenium. Technetium dissolves in
nitric acid, aqua regia, and concentrated sulfuric acid, but is
not soluble in hydrochloric acid of any strength. The element
is a remarkable corrosion inhibitor for steel. It is reported that mild carbon steels may be effectively protected by as little as
55 ppm of KTcO4 in aerated distilled water at temperatures up
to 250°C. This corrosion protection is limited to closed systems,
since technetium is radioactive and must be confined.
99Tc has a specific activity of 6.2 × 108 Bq/g. Activity of this
level must not be allowed to spread. 99Tc is a contamination
hazard and should be handled in a glove box. The metal is an
excellent superconductor at 11K and below. | | Characteristics | Technetium was the first element, not found on Earth, to be artificially produced by bombardingmolybdenum with deuterons.
The major characteristic of technetium is that it is the only element within the 29 transitionmetal-to-nonmetal elements that is artificially produced as a uranium-fission product innuclear power plants. It is also the lightest (in atomic weight) of all elements with no stableisotopes. Since all of technetium’s isotopes emit harmful radiation, they are stored for sometime before being processed by solvent extraction and ion-exchange techniques. The two longlivedradioactive isotopes, Tc-98 and Tc-99, are relatively safe to handle in a well-equippedlaboratory.
Since all of technetium’s isotopes are produced artificially, the element’s atomic weight(atomic mass units) is determined by which isotopes are selected for the calculation. | | Uses | Technetium is one of the few artificially produced elements that has practical industrial applications.One is that a very small amount (55-ppm) added to iron creates a corrosion-resistantalloy metal. This property is shared with many of the other transition metallic elements, but notwith other artificially produced elements that have higher atomic numbers and are radioactive.
A radioisotope of technetium is widely used in nuclear medicine. The patient is injectedwith saline solution containing Tc-99m (the superscript “m” means that the isotope is unstableand that its nuclei holds more energy than the regular Tc-99 nuclei into which it decays). Thismeans that the Tc-99m will start to emit energy and will finally decay and change to the regularnuclei of Tc-99 when injected into the patient. This energy is in the form of very penetratinggamma rays (a strong type of X-rays). The radioactive solution of Tc-99m may be combinedwith other elements that are absorbed by certain organs of the human body being diagnosedor treated. For instance, adding tin to the solution targets the red blood cells, whereas phosphorusin the solution concentrates the radioactive solution in heat muscles. The gamma raysare strong enough to expose an X-ray film that depicts the internal image of the organ underexamination. This procedure is safe because Tc-99m has a half-life of only 6.015 hours, andthe Tc-99 has a half-life of over 200,000 years. However, the radioactivity will be harmless inless than a day because the body rapidly eliminates the residual radioactive solution.
Technetium is also used as an alloy metal to produce super-strong magnets that are supercooledto near absolute zero to improve their efficiency. Powerful magnets are used in imagingequipment and possibly in future magnetic driven trains. Its radioactivity makes it useful as atracer in the production of metals and tracing flowing fluids in pipelines. | | Uses | Minute quantities of TcO4- ion exert remarkable inhibition of the corrosion of soft iron in neutral aqueous solution: Cartledge, J. Am. Chem. Soc. 77, 2658 (1955). | | Preparation | Technetium isotopes are prepared by bombardment of molybdenum with protons and neutrons. A few nuclear reactions are shown for the three longlived isotopes:
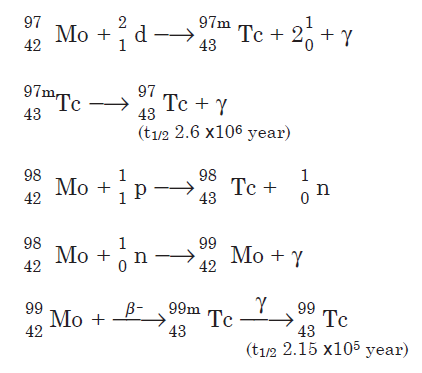
Technetium-99 also is a fission product of uranium-235.
Pure technetium metal may be prepared by reducing ammonium pertechnate, NH4TcO4, with hydrogen at high temperatures. Hydrogen reduction at about 200°C first forms the oxide, TcO2, which is reduced to Tc metal at 600 to 800°C. | | Definition | technetium: Symbol Tc. A radioactivemetallic transition element;a.n. 43; m.p. 2172°C; b.p. 4877°C. Theelement can be detected in certainstars and is present in the fissionproducts of uranium. It was firstmade by Carlo Perrier and EmilioSegré (1905–89) by bombardingmolybdenum with deuterons to givetechnetium–97. The most stable isotopeis technetium–99 (half-life 2.6 ×106 years); this is used to some extentin labelling for medical diagnosis.There are sixteen known isotopes.Chemically, the metal has propertiesintermediate between manganeseand rhenium. | | Definition | A transition metal that does not occur naturally on Earth. It is produced artificially by bombarding molybdenum with neutrons and also during the fission of uranium. It is radioactive. Symbol: Tc; m.p. 2172°C; b.p. 4877°C; r.d. 11.5 (est.); p.n. 43; r.a.m. 98.9063 (99Tc); most stable isotope 98Tc (half-life 4.2 × 106 years). | | Hazard | The hazards of technetium are the same as for all radioactive elements. Excessive exposureto radiation can cause many kinds of tissue damage—from sunburn to radiation poisoningto death. |
| | technetium Preparation Products And Raw materials |
|