- Ym-443
-

- $10.00 / 1kg
-
2024-04-02
- CAS:773092-05-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons
|
| | Acotiamide hydrochloride trihydrate Basic information |
| Product Name: | Acotiamide hydrochloride trihydrate | | Synonyms: | N-(2-(Diisopropylamino)ethyl)-2-((2-hydroxy-4,5-dimethoxybenzoyl)amino)-1,3-thiazole-4-carboxyamide monohydrochloride trihydrate;Unii-nmw7447A9a;Ym-443;AcotiaMide hydrochloride trihydrate;Acotiae;4-Thiazolecarboxamide, N-[2-[bis(1-methylethyl)amino]ethyl]-2-[(2-hydroxy-4,5-dimethoxybenzoyl)amino]-,hydrochloride,hydrate(1:1:3);Acofide trihydrate;N-{2-[bis(1-methylethyl)amino]ethyl}-2-[(2-hydroxy-4,5-dimethoxyphenylcarbonyl)amino] | | CAS: | 773092-05-0 | | MF: | C21H31ClN4O5S | | MW: | 487.013 | | EINECS: | 1592732-453-0 | | Product Categories: | Inhibitors;API;773092-05-0 | | Mol File: | 773092-05-0.mol | 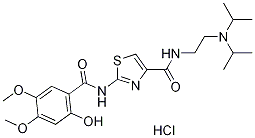 |
| | Acotiamide hydrochloride trihydrate Chemical Properties |
| Melting point | 193-195o C | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly, Heated, Sonicated), DMSO (Slightly) Methanol (Slightly) | | form | Solid | | color | Pale Yellow |
| | Acotiamide hydrochloride trihydrate Usage And Synthesis |
| Description | In June 2013, the Ministry of Health, Labor and Welfare in Japan approved acotiamide (also referred to as Z-338 and YM443), for the treatment of postprandial fullness, upper abdominal bloating, and early satiation due to functional dyspepsia (FD). Acotiamide enhances acetylcholine release from enteric neurons through selective muscarinic acetylcholine receptor M1, M2 antagonism, and inhibition of AChE, thereby enhancing gastric emptying and gastric accommodation. Acotiamide inhibits the acetylcholine-induced Ca2+-activated Cl-current with an IC50 of 1.8 μM in oocytes expressing muscarinic M1 receptors and in oocytes expressing muscarinic M2 receptors, acotiamide inhibited the acetylcholine-induced K+ currents with an IC50 of 10.1 μM. However, studies in conscious dogs, guinea-pig gastric muscle strips and assays with human cholinesterase indicate that acotiamide inhibits AChE with a Ki of 610 nM suggesting that acotiamide stimulates gastric motility mainly by inhibiting AChE activation. In a restraint stress-induced rat model, acotiamide significantly improved delayed gastric emptying and feeding inhibition but did not affect normal gastric emptying or feeding in intact rats. A synthetic route to acotiamide that employs pyridine hydrochloride to selectively cleave a 2-substituted methyl ether from a 2,4,5-trimethoxy benzamide intermediate, as a key step, has been reported. | | Originator | Zeria Pharmaceutical (Japan) | | Uses | Acofide Trihydrate is a gastrointestinal prokinectic agent used in the treatment of functional malnutrition. A tablet for the treatment of functional dyspepsia. | | Brand name | Acofide | | Synthesis | Commercial 3,4,5-trimethoxybenzoic acid was first converted
to the corresponding acid chloride, which was isolated
by co-distillation with hexane. In refluxing dichloroethane (DCE),
the acid chloride was coupled with the commercially available
thiazole amine to give the desired amidothiazole in 89% yield.
From this intermediate, amide linkage, selective demethylation of
the 2-methoxy group, salt formation, and recrystallization were
accomplished in the following sequence: the thiazole ester was
reacted with N,N-diisopropyl ethylenediamine in DMA at elevated
temperatures. Upon cooling, the mixture was dissolved in
n-butanol and washed with aqueous sodium hydroxide.
Subsequent treatment with HCl gas in isopropanol gave the
corresponding HCl salt as crystals that could be collected by filtration.
The product obtained was further crystallized from 4:1 isopropanol
and water to give the desired product acotimide (I) as
the hydrochloride trihydrate in 71% yield. 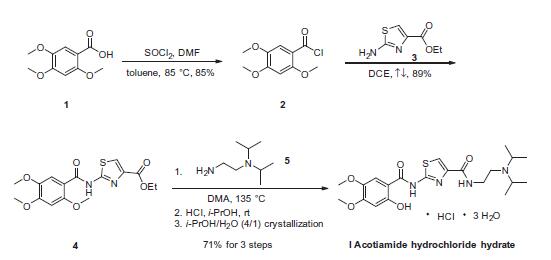
|
| | Acotiamide hydrochloride trihydrate Preparation Products And Raw materials |
|