
Aluminum fluoride NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-09-11 |
Product Details
| Product Name: Aluminum fluoride | CAS No.: 7784-18-1 |
| EC-No.: 232-051-1 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/09/11 |
| CAS: | 7784-18-1 |
| MF: | AlF3 |
| MW: | 83.98 |
| EINECS: | 232-051-1 |
| Product Categories: | metal halide;Inorganics;Inorganic Fluorides;Aluminum Salts;AluminumMetal and Ceramic Science;Crystal Grade Inorganics;Metal and Ceramic Science;Salts |
| Mol File: | 7784-18-1.mol |
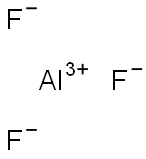 | |
| Aluminum fluoride Chemical Properties |
| Melting point | 1290 °C (lit.) |
| Boiling point | 1291 °C |
| density | 3.1 g/mL at 25 °C (lit.) |
| Fp | 1250°C |
| solubility | Sparingly soluble in acids and alkalies. Insoluble in Acetone. |
| form | powder |
| color | White to light gray |
| Specific Gravity | 2.882 |
| Water Solubility | SLIGHTLY SOLUBLE |
| Sensitive | Hygroscopic |
| Sublimation | 1250 ºC |
| Merck | 14,339 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2 mg/m3; TWA 2.5 mg/m3 |
| CAS DataBase Reference | 7784-18-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Aluminum trifluoride(7784-18-1) |
| EPA Substance Registry System | Aluminum trifluoride (7784-18-1) |
| Aluminum fluoride Usage And Synthesis |
| Description | Aluminum fluoride is in the form of white crystalline solid or a white powder. It occurs naturally as minerals rosenbergite and oskarssonite.1 It can also be prepared synthetically. |
| Synthesis | Aluminum fluoride are produced by methods in the following: 1) Fluosilicic acid with aluminum hydroxide2 H2SiF6 + 2 Al(OH)3 → 2 AlF3 + SiO2 + 4 H2O (I) The reaction is exothermal and proceeds in several steps. It can be described by the following three reactions: 3H2SiF6 + 2 Al(OH)3 → Al2(SiF6)3 + 6 H2O (II) Al2(SiF6)3 + 6 H2O → 2 AlF3 + 3 SiO2 + 12 HF (III) 12 HF + 4 Al(OH)3 → 4 AlF3 + 12 H2O The reaction is carried between 70°C and 100°C. The concentration of fluosilicic acid can be as high as 35 wt% in a water solution. As the produced aluminum fluoride solution is metastable and the trihydrate begins to crystalize out quickly at temperature around 90°C, precipitated solid silica must be removed as quickly as possible. 2) Al2O3 with aqueous hydrofluoric acid3 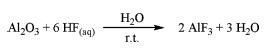 Hydrofluoric acid is added dropwise under vigorous stirring at room temperature into Al2O3 suspension. Subsequently the solid oxide is completely dissolved. The resulting clear solution is stirred for days at room temperature. During this period, the white solid precipitates are separated from the solvent, washed with water, and consequently dried. After treatment in a muffle furnace at the desired calcination temperature, aluminum fluoride is obtained. 3) Aluminum alkoxide with non-aqueous HF solution4 Aluminum alkoxide, Al(OR)3 (R = Me, Et, iPr, or tBu), reacts with non-aqueous HF solution in an organic solvent. The reaction is carried in a sol-gel state. After aging, a solid is formed which settles. The solid is separated by centrifugation and dried under vacuum. Gas phase fluorination of the dried gel is then applied to obtain aluminum fluoride. |
| Application | Aluminum fluoride is one of the most important additives in the industrial production of aluminum. The usage of aluminum fluoride can lower the melting point of cryolite and better the physical and chemical properties of the electrolyte. In the aluminum production, aluminum oxide is dissolved in a solution of cryolite. By passing an electrical current through the solution, aluminum is produced. Nevertheless, cryolite solution melts at about 1000°C. When aluminum fluoride is added, electrolysis can happen in the cryolite solution at a temperature 40-60°C lower, reducing the required amount of energy to produce aluminum.5 Aluminum fluoride is used as a barrier layer to retard oxidation of aluminum mirrors.6 It is used as a flux in ceramic glazes and enamels, in the manufacture of aluminum silicate, and as a catalyst. Aluminum fluoride complexes with proteins can be used to study the mechanistic aspects of phosphoryl transfer reactions in biology, which are of fundamental importance as phosphoric acid anhydrides such as ATP and GTP control most of the reactions involved in metabolism, growth and differentiation.8 Aluminum fluoride, together with zirconium fluoride, is used to produce fluoroaluminate glasses. In agriculture, aluminum fluoride can be used to inhibit fermentation. Physical vapor deposited aluminum fluoride can be used as a low index optical thin film in situations when far UV transparency is required.1 |
| Toxicological studies | Oral animal lethal dose (LD50) of aluminum fluoride is 0.1 g/kg. Aluminum fluoride is less toxic than most fluorides due to its slight water solubility. Exposure to high concentration of aluminum fluoride causes hypocalcemia. Inhalation and ingestion of aluminum fluoride result in typical symptoms of fluoride poisoning. Symptoms of severe poisoning include shortness of breath, congestion of the lungs, muscle spasm, and convulsions.8 Acute (short-term) toxic effects may include the death of animals, birds, or fish and death or low growth rate in plants. Acute effects are observed in 2 to 4 days after exposure of animals or plants to aluminum fluoride. Chronic (long-term) toxic effects may include shortened life span, reproductive problems, lower fertility, and changes in appearance or behavior in exposed animals. |
| Safeties | Aluminum fluoride is a non-combustible solid. But it is incompatible with many other commodities including chemically active metals (e.g. potassium and sodium), acid, and acid fumes. Contact can cause fire or explosion. Upon heating, toxic fumes of fluoride, including extremely toxic hydrogen fluoride, can be emitted. |
| References |
|
| Chemical Properties | Aluminum fluoride is a white, odorless powder or granule. |
| Chemical Properties | Aluminum fluoride, AlF3, is an anhydrous crystalline powder with a melting point of 1291 "C. Aluminum fluoride (hydrated), AlF3·31/2H20, is a white crystalline powder that is insoluble in water. |
| Uses | Production of aluminum to lower the melting point and increase the conductivity of the electrolyte, flux in ceramic glazes and enamels, manufacture of aluminum silicate, catalyst. |
| Uses | Aluminum fluoride is used to produce lowmelting aluminum metal, as a flux in ceramic glazes and white enamels, and as a catalyst in chemical reactions. |
| Uses | In ceramics, as flux in metallurgy, in aluminum manufacture, as inhibitor of fermentation, as catalyst in organic reactionsAluminum fluoride is used in the smelting process to lower the melting point of electrolytes, production of aluminum silicates, refining of aluminum scarp, production of specialty refractory products, and in glass industry as filler. It is used as flux ingredient to remove magnesium. |
| General Description | Odorless white powder or granules. Denser than water. Solubility in water at 25°C equals 0.559 g / 100 mL. |
| Air & Water Reactions | Slightly soluble in water |
| Reactivity Profile | Aluminum fluoride when heated to sublimation condition, emits toxic fumes of fluoride [USCG, 1999]. |
| Hazard | Strong irritant to tissue. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $10.00/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-10-28 | |
| $48.00/1KG |
Shijiazhuang tongyang Import and Export Co., LTD
|
2021-08-10 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-11 | ||
| $1.00/1g |
Cangzhou Wanyou New Material Technology Co.,Ltd
|
2019-08-22 | ||
| $1.00/1kg |
VIP6Y
|
Career Henan Chemical Co
|
2018-12-20 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-09 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-06-27 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 China
China