
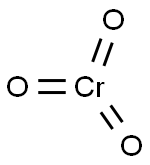
Chromium(VI) oxide NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-08-29 |
Product Details
| Product Name: Chromium(VI) oxide | CAS No.: 1333-82-0 |
| EC-No.: 215-607-8 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/08/29 |
| CAS: | 1333-82-0 |
| MF: | CrO3 |
| MW: | 99.99 |
| EINECS: | 215-607-8 |
| Product Categories: | Inorganic chemistry;Dye;INORGANIC & ORGANIC CHEMICALS;Inorganics;metal oxide;catalytic agent,chromium compound, wood preservation, medicine, catalyzer , printing, oxidant, oxidation acceleration. |
| Mol File: | 1333-82-0.mol |
 | |
| Chromium(VI) oxide Chemical Properties |
| Melting point | 196 °C (dec.)(lit.) |
| Boiling point | 330 °C |
| density | 2.7 |
| vapor pressure | 0Pa at 25℃ |
| Fp | 250°C |
| storage temp. | Store below +30°C. |
| solubility | 1.667g/l |
| pka | -0.61[at 20 ℃] |
| form | macroporous |
| Specific Gravity | 2.7 |
| color | Reddish-violet |
| PH | <1 (100g/l, H2O, 20℃) |
| Water Solubility | Highly soluble |
| FreezingPoint | 170~172℃ |
| Sensitive | Hygroscopic |
| Merck | 14,2235 |
| Exposure limits | ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin) OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
| Stability: | Stable. Strong oxidizer. Reacts with most organic material in a violent and often explosive fashion. Moisture sensitive. |
| InChIKey | WGLPBDUCMAPZCE-UHFFFAOYSA-N |
| CAS DataBase Reference | 1333-82-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Chromium trioxide(1333-82-0) |
| EPA Substance Registry System | Chromium(VI) trioxide (1333-82-0) |
| Chromium(VI) oxide Usage And Synthesis |
| Chemical properties | It appears as dark red rhombic crystals or purple-red flakes. Soluble in water, alcohol, sulfuric acid and ether, do not dissolve in acetone. |
| Uses | Chromium(VI) oxide is used for chromium plating; copper stripping; as an oxidizing agent for conversion of secondary alcohols into ketones (Jones oxidation); as a corrosion inhibitor; in purification of oil; and in ‘chromic mixtures’ for cleaning laboratory glassware. 1. It is used for electroplating and the manufacturing of high-purity metal chromium, dyes, synthetic rubber and refined oil, and so on 2. Chromium(VI) oxide is the main raw material of electroplating chrome. During the electroplating chrome production, chromium content has a large fluctuation within the range of 50 to 500 g/L. It should be mainly controlled of the appropriate temperature and current density. It can be obtained in the smooth plating of the bright coating. Its solution can also be used for zinc coating passive film, to improve the protection performance. 3. It is mainly used for electroplating industry as the raw materials of electroplating chromium used in making bicycles, sewing machines, watches, meters, flashlights and household hardware as well as for the production of high-purity metal chromium. It is the raw materials for the manufacture of chromium oxide green and zinc chrome yellow; is also the raw materials for the production of low-temperature shift catalyst, high-temperature shift catalyst and high-pressure methanol synthesis catalyst. It can be used as mordant in wool fabric dyeing; as oxidant in printing and dyeing industry; also used for wood preservative. 4. The solution is used for chrome plating 5. It is used as analytical reagent and strong oxidant, also used in the manufacture of chromate 6. It is strong oxidizing agent; for determination of carbon and phosphorus; the purification of oil and ethylene; manufacturing of chromium salt. 7. Oxidants, glass coloring, raw materials for the manufacturing of oxidation catalyst. |
| Reaction | Chromium(VI) oxide decomposes to chromium(III) oxide liberating oxygen when heated at 250°C: 4Cr2O3→ 2CrO3 + 3O2 The red oxide is the acid anhydride of two acids, namely, chromic acid, H2CrO4 or CrO2(OH)2 and the dichromic acid H2Cr2O7. Both the chromic and dichromic acids exist only in the aqueous solution and have not been isolated from the solution. Dissolution of CrO3 in water produces H+ ion along with dichromate ion, Cr2O72– as follows: 2CrO3 + H2O → 2H+ + Cr2O72(red-orange dichromic acid) The aqueous solution of CrO3 is, therefore, strongly acidic because of this proton release. The Cr2O72– ion in the aqueous solution is susceptable to further decomposition, forming chromate ion: Cr2O72– → CrO42– + CrO3 In the above reaction the equilibrium, however, lies far to the left. Therefore the chromium(VI) oxide solution also contains trace amounts of chromate ion, CrO42–. Addition of stoichiometric amounts of caustic soda or caustic potash yields orange dichromate salt which can be crystallized from the solution. Cr2O72–+ 2Na+ → Na2Cr2O7 If excess base is added to this solution, it turns yellow, and yellow chromate salt may crystallize out. Thus, as mentioned above, in an aqueous solution of CrO3, there is an equilibrium between two Cr6+ species, namely, the chromate and dichromate ions: 2CrO42– + 2H+ → Cr2O72– + H2O Kc = 4.2x1014 The addition of base (OH–) shifts the equilibrium to the left while acidification of the solution shifts the equilibrium to the right in favor of Cr2O72–. In other color/pH relations, red CrO3 is acidic, green Cr2O3 is amphoteric and the black CrO is basic in nature. In acid medium chromic acid oxidizes secondary alcohols to ketones: R2CHOH + 2H2CrO4 + 6H+ 3R2C=O + 2Cr3+ + 8H2O The reaction usually is carried out in acetone or acetic acid. Chromium is reduced from +6 to +3 oxidation state. Reaction with hydrochloric acid yields chromyl chloride: CrO3 + 2HCl → CrO2Cl2 + H2O A similar reaction occurs with HF to yield chromyl fluoride CrO2F2. However, fluorination with F2 yields the oxohalide, CrOF4. |
| Production method | Sulfuric acid method The sodium dichromate solution (70 ° Bé) and 98% sulfuric acid were separately added into the reactor with stirring device, stirred and mixed, and heated and melted by direct fire to produce chromic anhydride and sodium bisulfate. Sulfuric acid by the theoretical amount of about 102% were added, the reaction end temperature should be controlled at 200~205 ℃. When all the materials are melted, stop heating and stirring, leaving the material stratified so the light sodium bicarbonate floated in the upper while the heavier molten chromic anhydride was discharged from the bottom valve of the reactor, further cooled, and solidified for tablets through the drum knotting machine, leading to chromic anhydride products. Its Na2Cr2O7 + 2H2SO4 → 2CrO3 + 2NaHSO4 + H2O The molten sodium bisulfate contains a small amount of chromic anhydride and can be solidified into solid to be recovered after cooling; the melted sodium bisulfate can be directly sent into the water (be careful to prevent spill) to dissolve into a solution of concentration of 40~42 ° Bé for the manufacture of basic chromium sulfate. Its production methods include sulfuric acid, nitric acid, fluosilicic acid, electrolysis. Among them, commonly used is sulfuric acid method. As follows: The sodium dichromate solution (70 ° Bé) and sulfuric acid (98%) were mixed in a reactor and heated and melted. After all the 190 °C solid matter is melted, the heating is stopped. Stirring of the material is stopped. The chromic anhydride deposited in the lower layer was placed into the rum knotting machine from the bottom of the reactor and coagulated. Pack it to get the final product. |
| Toxicity | Chromium compounds have local simulating effect on the skin and mucous membrane, being able to cause ulcers. Inhalation of this product can cause nasal septal aerosol cartilage perforation, so that respiratory organs are damaged, and even cause pulmonary sclerosis. The general toxicity appears as the damage in the liver, kidney, gastrointestinal tract and cardiovascular system. It may also be occurred of conjunctivitis, even causing blind upon in contact with eyes. Upon nasal mucosa injury, it can be first washed with soap, and then apply ointment to the nose after work and before going to bed. When there is ulceration, it should be stopped to be in contact with chromium for 2 months. When splashed on the skin, immediately rinse with water for 15 min. When there are eczema and dermatitis, it should be locally applied of lotion, dusting and non stimulating ointment. If accidentally fall into the eyes, immediately rinse with water for 15 min or more, and then drop cod liver oil, and then drop 30% of the sulfa acetyl solution. In severe cases, the patients should be immediately sent to hospital for treatment. The maximum allowable concentration (according to CrO3 dollars) is 0.01mg/m3. Production equipment should be completely closed and the workplace to be well-ventilated, so that the concentration of chromium in the air can be maintained at a very low level. Production workers should wear overalls, wear masks and use rubber aprons and latex gloves. If there are aerosols in the air, the worker should wear gas masks. At the end of the work, they must take shower with the skin damage place being coated with protective ointment. It should be conducted of a medical examination in advance and rechecked once every two years; add an extra examination every three months. |
| Hazards & Safety Information | Category: Oxidant Toxicity classification : highly toxic Acute toxicity : Oral-rat LD50: 80 mg/kg; oral-mouse LD50: 127 mg/kg Explosive and hazardous properties: mixed heating, impact and friction with reducing agent, sulfur and phosphorus can cause explosion Flammability hazard characteristics: Combustible upon coming across combustible stuff Storage and transportation characteristics: Treasury: ventilated, low temperature and dry; store it separately from combustible, organic and reducing agent. Fire extinguishing agent: Mist Occupational Standard: TWA 0.05 mg (Cr)/m3; STEL 0.1 mg (Cr)/m3 |
| Description | Chromium hydroxide (Cr2O(OH)4) is a bright bluish-green pigment prepared by the calcinations of bichromate with boric acid at 500°C. The mass during cooling is hydrolyzed with water, which yields the hydrate. |
| Chemical Properties | Chromium trioxide is a dark-red crystalline substance. It is odorless |
| Physical properties | Dark-red crystals, flakes or granular powder; bipyramidal prismatic system; density 2.70 g/cm3; melts at 197°C; decomposes on further heating; highly soluble in water, 61.7 g and 67 g/100 mL at 0°C and 100°C, respectively; soluble in sulfuric and nitric acids. |
| Uses | Chromium(VI) oxide is widely used in chromium electroplating, and as protective coatings in corrosion/oxidation resistance of metals. It is also employed in CDs and DVDs. It is useful as an oxidant in Jones oxidation in acetic acid or acetone. It is used in the oxidation of primary alcohols into carboxylic acids, and secondary alcohols into ketones. With phosphoric acid, it is used as a stripping agent for anodic coatings of all types and in the production of syntheric rubies. |
| Definition | chromium trioxide: A redcompound, CrO3; rhombic; r.d. 2.70;m.p. 196°C. It can be made by carefuladdition of concentrated sulphuricacid to an ice-cooled concentratedaqueous solution of sodium dichromatewith stirring. The mixture isthen filtered through sintered glass,washed with nitric acid, then dried at120°C in a desiccator. Chromium(VI) oxide is an extremelypowerful oxidizing agent,especially to organic matter; it immediatelyinflames ethanol. It is anacidic oxide and dissolves in water toform ‘chromic acid’, a powerful oxidizingagent and cleansing fluid forglassware. At 400°C, chromium(VI)oxide loses oxygen to givechromium(III) oxide. |
Product picture

Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $10.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-07-28 | ||
| $1378.00/1Tons |
VIP2Y
|
Hebei Dangtong Import and export Co LTD
|
2023-03-17 | |
| $1.00/1kg |
VIP2Y
|
Hebei Duling International Trade Co. LTD
|
2022-12-09 | |
| $9.90/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-09-30 | |
| $1.10/1g |
VIP4Y
|
Dideu Industries Group Limited
|
2021-07-21 | |
| $10.50/1KG |
VIP3Y
|
Hebei Guanlang Biotechnology Co,.LTD
|
2021-07-13 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $0.00/1KG |
VIP5Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-05-02 | |
| $1.00/1KG |
VIP6Y
|
Career Henan Chemical Co
|
2019-07-11 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-09 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 China
China