
HYDRAZINE NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-09-13 |
Product Details
| Product Name: HYDRAZINE | CAS No.: 302-01-2 |
| EC-No.: 206-114-9 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/09/13 |
| CAS: | 302-01-2 |
| MF: | H4N2 |
| MW: | 32.05 |
| EINECS: | 206-114-9 |
| Product Categories: | Organic Building Blocks;Water Ttreatment Chemicals;Aromatic Hydrazides, Hydrazines, Hydrazones and Oximes;HydrazinolysisEssential Chemicals;Chemical Deglycosylation;Deglycosylation Strategies;Hydrazines;Nitrogen Compounds;Reagent Grade;Routine Reagents |
| Mol File: | 302-01-2.mol |
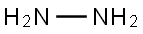 | |
| HYDRAZINE Chemical Properties |
| Melting point | 1,4°C |
| Boiling point | 65 °C |
| density | 1.011 g/mL at 25 °C |
| vapor density | >1 (vs air) |
| vapor pressure | 5 mm Hg ( 25 °C) |
| refractive index | n20/D 1.47(lit.) |
| Fp | −4 °F |
| storage temp. | 2-8°C |
| pka | pK1 (25°): ~6.05 |
| form | Liquid |
| color | Clear colorless |
| Odor | Fishy or ammonia-like odor detectable at 3 to 4 ppm (mean = 3.7 ppm) |
| explosive limit | 99.99% |
| Water Solubility | miscible with H2O and the following alcohols: methyl, ethyl, propyl, isobutyl [MER06] |
| Merck | 13,4789 |
| BRN | 878137 |
| Exposure limits | TLV-TWA (skin) 1 ppm (1.3 mg/m3 ) (MSHA and OSHA), 0.1 ppm (ACGIH). |
| Stability: | Stability May be an explosion hazard, particularly if heated. Incompatible with sources of ignition, light, shock, strong oxidizing agents, strong acids, metal oxides, nitrous oxide, hydrogen peroxide, most common metals, organic materials, porous materials such as wood, paper, asbestos, soil or rust. Many types of metal may cause rapid d |
| LogP | -0.16 at 20℃ |
| CAS DataBase Reference | 302-01-2(CAS DataBase Reference) |
| IARC | 2A (Vol. 4, Sup 7, 71, 115) 2018 |
| EPA Substance Registry System | Hydrazine (302-01-2) |
| HYDRAZINE Usage And Synthesis |
| Chemical Properties | Hydrazine is a colorless flammable liquid with strong toxicity and instability, and combines easily in the air with water molecules to form hydrazine hydrate. It is also very important for industrial uses such as rocket fuel, photographic development, and in fuel cells. Hydrazine is the simplest diamine in its class of compounds and may be thought of as derived from ammonia by replacement of a hydrogen atom by the – NH2 group. The hydrated hydrazine, N2H4.H2O was first prepared by Curtius in 1887. The anhydrous hydrazine as a water free base was prepared by De Bruyn for the first time. Preparation of hydrazine by the oxidation of NH3 with hypochlorite – a process that became the chief commercial method of manufacture was first demonstrated by Raschig. |
| Uses | Hydrazine is mostlyed used as a blowing agent in preparing polymer foams. It is mainly used as rocket fuels, boiler water treatments, chemical reactants, medicines, and in cancer research. |
| Production | The industrial production of hydrazine always applies Raschin method. In October 1981, the French company Jürgen-Coleman successfully studied the imine hydrogen peroxide oxidation method, which is a promising method of production. Raschin uses ammonia and sodium hypochlorite as raw materials, followed by chlorination and amination to obtain hydrazine: ammonia and sodium hypochlorite are sent into the reactor in 1: 3 (molar ratio) with reaction generating chloramines. Chloramine can react with anhydrous ammonia to generate hydrazine in the hydrazine reactor. We can also use urea as the raw material to have it reacted with sodium hypochlorite-sodium hydroxide solution in the presence of a potassium permanganate catalyst (see hydrazine hydrate, [7803-57-8]). The dehydrating agent method mixes caustic soda and 50% ~ 54% hydrazine hydrate (the mass ratio is 10: 8), gradually send nitrogen to remove air and heats the alkaline temperature to 118 DEG C. After the caustic soda is completely dissolved, cool to around 60℃, apply vacuum distillation to a distillate containing hydrazine content of 90% to 94%, followed by fractional distillation to remove water, condensation to obtain 98% to 99.5% of anhydrous hydrazine. Extraction dehydration method: hydrazine hydrate solution can subject to fractionation to distill out of water to until the water and hydrazine become azeotropic (68% hydrazine); the solution is subject to secondary fractionation; add aniline to change its boiling point and distill the aniline and water off from the water; the recovered aniline can be for recycling use; the secondary fractionation solution is further subject to three times fractionation to obtain anhydrous hydrazine. |
| Description | Hydrazine sulphate, hydrobromide and hydrochloride have been reported to be occupational sensitizers, mainly in soldering flux. |
| Chemical Properties | HYDRAZINE, colorless, fuming liquid, decomposes when heated above 350 °C at atmospheric pressure into N2 and NH2, also decomposes in presence of a catalyst (e.g., platinum) into N2 and NH3. Hydrazine burns when ignited in air with a violet-colored flame. The compound is soluble in all proportions with H2O and is soluble in alcohol. Hydrazine forms a hydrate with one molecule of H2O. Upon moderate heating or in a vacuum, the hydrate yields hydrazine and H2O. Hydrazine is a base slightly weaker than NH4OH. |
| Chemical Properties | colourless oily liquid |
| Physical properties | Colorless, mobile, fuming liquid; ammoniacal odor; density 1.0045 g/mL at25°C; refractive index 1.46044 at 22°C; solidifies at 2°C to a white crystallinesolid; boils at 113.5°C; flash point 52°C; burns with a violet flame; vapor pres-sure 14.4 torr at 25°C; critical temperature 379.85°C; critical pressure 145atm; surface tension 66.67 dyne/cm at 25°C; dielectric constant 51.7 at 25°C;viscosity 0.876 centipoise at 25°C; very soluble in water; forms an azeotropewith water at molar composition of 58.5% hydrazine: 41.5% water (71.48%:28.52% by weight), the azeotrope with water boils at 120.5°C; forms hydrazinehydrate at 1:1 molar concentration in water; soluble in alcohols and other polar solvents; pKa 8.1 at 25°C. |
| History | Hydrazine was isolated first as a sulfate salt by Curtius in 1887. Earlier, in1875, Fischer prepared and identified the organic derivatives of hydrazine.Raschig in 1906 prepared hydrazine by hypochlorite oxidation of ammonia. Hydrazine and its derivatives have numerous commercial applications. Itwas used initially as rocket propellant. During World War II, it was used as afuel for rocket-powered fighter planes. However, the most important applications of hydrazine and its derivatives at present are: as blowing agents; forinsect control; in pharmaceuticals; in water treatment; and in fuel cells.Hydrazine derivatives release nitrogen on decomposition, producing foamingaction in polymers to form pores or cells. A large number of hydrazine derivatives are used in agricultural applications as fungicides, herbicides, and pesticides for weed and pest control. A few hydrazide drugs, such as isoniazid [54-85-3] are used extensively for treating tuberculosis. Other applications ofhydrazine include its use in fuel cells; and in wastewater treatment forremoval of iron; iron removal from hot-water heating systems; reduction ofred iron oxide rust into magnetite; and for removal of oxygen to protectagainst corrosion. It also is used in electrolytic plating of metals on glassesand as a reducing agent. Several hydrazine derivatives are used in azo dyes;as coupling agents in color photography; and in explosives and ammunitionprimers. |
| Uses | Hydrazine is used as a high-energy rocket fuel, as a reducing agent, and for preparing organic hydrazine derivatives. The propellant grade of commercial hydrazine is more than 97.7% active. It is also used as an oxygen scavenger in boiler water. Hydrazine has also been used as an experimental drug for treating tuberculosis and sickle cell anemia. |
| Uses | Reducing agent for many transition metals and some nonmetals (arsenic, selenium, tellurium), as well as uranium and plutonium; corrosion inhibitor in boiler feedwater and reactor cooling water; waste water treatment; electrolytic plating of metals on glas |
| Uses | Hydrazine is a highly reactive base and powerful reducing agent. It acts as an oxygen scavenger and is highly reactive with other chemicals. Hydrazine has a number of uses including, as a chemical precursor to blowing agents (e.g., azodicarbonamide and azobisisobutyronitrile), in the organic synthesis of pharmaceuticals and pesticides (e.g., isoniazid, fluconazole, and 3-amino-1,2,4-triazole), as a missile and rocket propellant (e.g., used in the F-16 fighter), as a gas-forming agent in air bags (e.g., sodium nitrite), as a corrosion inhibitor and reducing agent in large industrial boilers, and as a fuel source in fuel cells. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/100KG |
VIP6Y
|
Jinan Finer Chemical Co., Ltd
|
2022-05-12 | |
| $0.00/50mg |
VIP1Y
|
Xian confluore Biological Technology Co., Ltd.
|
2024-03-29 | |
| $20.00/25kg |
VIP1Y
|
Ouhuang Engineering Materials (Hubei) Co., Ltd
|
2024-04-15 | |
| $10.00/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-04-23 | |
| $0.10/1KG |
VIP5Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-01-02 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 China
China