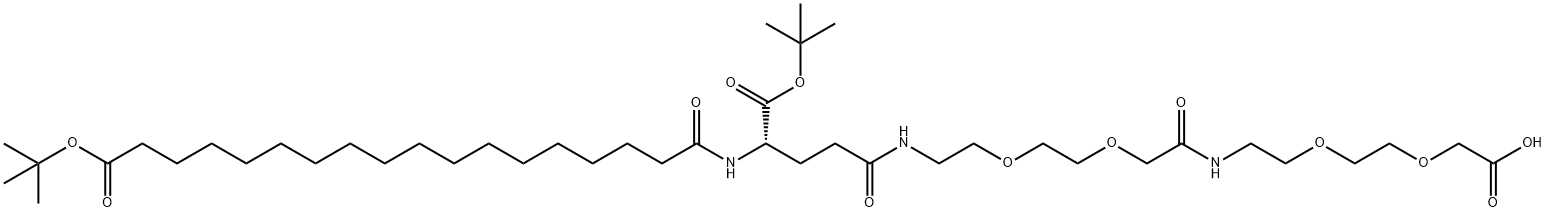
(S)-22-(Tert-butoxycarbonyl)-10,19,24-trioxo-3,6,12,15-tetraoxa-9,18,23-triazahentetracontane-1,41-dioic acid: properties and applications
Dec 1,2023
General Description
(S)-22-(Tert-butoxycarbonyl)-10,19,24-trioxo-3,6,12,15-tetraoxa-9,18,23-triazahentetracontane-1,41-dioic acid, also known as semaglutide side chain, is a synthetic peptide with a molecular weight of 846.1 Da. It possesses important properties such as antioxidation, drug delivery, and biocompatibility, making it highly valued in biomedical research. This compound exhibits antioxidative characteristics, protecting cells from oxidative stress and promoting cellular health. Furthermore, its unique molecular structure leads to strong biocompatibility, resulting in widespread applications in biomedical engineering and drug formulation. Moreover, (S)-22-(Tert-butoxycarbonyl)-10,19,24-trioxo-3,6,12,15-tetraoxa-9,18,23-triazahentetracontane-1,41-dioic acid plays a crucial role in organic synthesis and chemical biology. It serves as a key building block for modifying insulin molecules, leading to improved pharmacokinetic profiles and enhanced stability. Additionally, it is an essential intermediate in the synthesis of somatostatin analogs, offering promise for more effective treatment options for neuroendocrine disorders. Its contribution to enhancing the efficiency and quality of related drugs underscores its significance in advancing the research and development of pharmaceuticals for these conditions.

Figure 1. (S)-22-(Tert-butoxycarbonyl)-10,19,24-trioxo-3,6,12,15-tetraoxa-9,18,23-triazahentetracontane-1,41-dioic acid
Properties
(S)-22-(Tert-butoxycarbonyl)-10,19,24-trioxo-3,6,12,15-tetraoxa-9,18,23-triazahentetracontane-1,41-dioic acid, also known as semaglutide side chain, is a synthetic peptide with a molecular weight of 846.1 Da and an important structural unit in bioactive peptides, possessing multiple significant properties. Firstly, it exhibits antioxidative characteristics, effectively protecting cells from oxidative stress, thereby promoting cellular health and stability. Secondly, semaglutide side chain demonstrates excellent controllability and stability in drug delivery. It serves as a carrier for drugs, facilitating their release and delivery within the body, thereby enhancing drug efficacy and reducing side effects. Furthermore, due to its unique molecular structure, semaglutide side chain also displays strong biocompatibility, leading to its widespread application in the field of biomedical engineering and drug formulation. In summary, (S)-22-(Tert-butoxycarbonyl)-10,19,24-trioxo-3,6,12,15-tetraoxa-9,18,23-triazahentetracontane-1,41-dioic acid possesses important properties such as antioxidation, drug delivery, and biocompatibility, making it a highly regarded functional compound in the field of biomedical research. 1
Applications
(S)-22-(Tert-butoxycarbonyl)-10,19,24-trioxo-3,6,12,15-tetraoxa-9,18,23-triazahentetracontane-1,41-dioic acid, also known as t-Bu protected C18-diacid-yGlu-Ado-Ado-OH, is a versatile compound with significant applications in organic synthesis and chemical biology. One of its primary applications is in the synthesis of acylated insulin analogues. In the realm of pharmaceutical research, t-Bu protected C18-diacid-yGlu-Ado-Ado-OH plays a crucial role as a key building block for modifying insulin molecules to enhance their pharmaceutical properties. Specifically, it is used in the acylation of insulin analogues, introducing specific chemical functionalities to the insulin molecule. This modification can lead to improved pharmacokinetic profiles, enhanced stability, and altered physiological effects, all of which are valuable in developing novel therapeutic agents for managing diabetes and related metabolic disorders. The synthesis process involves dissolving the tert-butyl octadecandioyl-Glu(OEG-OEG-OH) in a suitable solvent, adding specific reagents, and conducting a reaction under optimized conditions. The resulting product serves as a vital intermediate for the synthesis of acylated insulin analogues. Furthermore, t-Bu protected C18-diacid-yGlu-Ado-Ado-OH also plays a crucial role as an intermediate in the synthesis of semaglutide, used in pharmaceutical applications. Somatostatin and its analogs have garnered significant interest in the medical field due to their diverse therapeutic potential, including the treatment of neuroendocrine tumors, acromegaly, and pancreatic disorders such as insulin-dependent diabetes. This specific intermediate, (S)-Boc-protected trioxatridecanedioic acid, is instrumental in the synthesis of somatostatin analogs for several reasons. Firstly, it serves as a key building block in the assembly of the peptide chain, ensuring precise construction and modification necessary for high purity and biological activity of the final pharmaceutical product. Additionally, this intermediate compound enables the synthesis of somatostatin derivatives with enhanced stability, prolonged half-life, and improved pharmacological properties by allowing for modifications at specific functional groups. In summary, (S)-22-(Tert-butoxycarbonyl)-10,19,24-trioxo-3,6,12,15-tetraoxa-9,18,23-triazahentetracontane-1,41-dioic acid's role as an intermediate in the synthesis of somatostatin analogs holds great promise for advancing the research and development of somatostatin-based pharmaceuticals. Its contribution to enhancing the efficiency and quality of related drugs underscores its significance in offering more effective treatment options for neuroendocrine disorders. 2,3
Reference
1. PubChem. COMPOUND SUMMARY: (S)-22-(tert-Butoxycarbonyl)-43,43-dimethyl-10,19,24,41-tetraoxo-3,6,12,15,42-pentaoxa-9,18,23-triazatetratetracontanoic acid. National Library of Medicine, PubChem CID: 87275422.
2. Niu X, Nong S, Zhang X, Li X, Wang C, Li W, Zhou T. Design and evaluation of novel thrombin-based GLP-1 analogs with peptidic albumin binding domain for the controlled release of GLP-1. RSC Adv. 2020 Jan 29;10(8):4725-4732.
3. Janssen Pharmaceutica Nv. Macielag M, Patch RJ, Zhang R, Case MA, Rangwala SM, Leonard JN, Wall M, Chi E. Antibody-coupled cyclic peptide tyrosine tyrosine compounds as modulators of neuropeptide y receptors. US2018/117170[P], 2018, A1, Paragraph 0511.
- Related articles
- Related Qustion
- Reaction / Application on Synthetic Works of this Intermediate Nov 20, 2019
(S)-22-(tert-butoxycarbonyl)-43,43-dimethyl-10,19,24,41-tetraoxo-3,6,12,15,42-pentaoxa-9,18,23-triazatetratetracontanoic acid is an important organic intermediate to act as crosslinkers to functionalize molecules.
The results presented in this report may be useful for further studies evaluating the effects of the tested chromium(III) complexes on human body as antioxidants or dietary supplements substituting Cr(pic)3.....
Dec 1,2023APIp-Anisidine produced conjunctival injection, chemosis, and colorless diseharge. Miosis was observed from 15 min-2 hrs. Ocular effects disappeared in 1 eye at 72 hrs.....
Dec 1,2023APItBuO-Ste-Glu(AEEA-AEEA-OH)-OtBu
1118767-16-0You may like
tBuO-Ste-Glu(AEEA-AEEA-OH)-OtBu manufacturers
- tBuO-Ste-Glu(AEEA-AEEA-OH)-OtBu
-
- $2.00 / 1KG
- 2024-04-15
- CAS:1118767-16-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Octadecane(OtBu)-Glu(ɑ-OtBu)-AEEA-AEEA-OH
-
- $0.00 / 1g
- 2024-01-27
- CAS:1118767-16-0
- Min. Order: 1g
- Purity: >98%
- Supply Ability: 15kg per batch
- tBuO-Ste-Glu(AEEA-AEEA-OH)-OtBu
-

- $15.00 / 1KG
- 2021-07-13
- CAS:1118767-16-0
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton