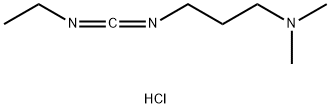
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: Synthesis method and uses
Apr 16,2024
Description
Carbodiimide reagents, such as 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl), N, N'-diisopropyl carbodiimide (DIC) and N, N'-dicyclohexylcarbodiimide (DCC), accelerate the formation reaction of esters, amides, and peptides, as condensing and dehydrating agents, which are often used for polynucleotide synthesis, anhydroxydation, lactonization, and esterification. Therefore, these reagents are quite significant in the field of biochemistry research.
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is a white crystalline powder that easily absorbs moisture. Its solubility in water is >20g/100ml and soluble in ethanol. Its relative molecular mass is 191.70, and its melting point is 110-114°C. The pH range when used is 4.0-6.0, often used in conjunction with N-hydroxysuccinimide (NHS) or N-hydroxysulfosuccinimide to improve coupling efficiency.
Uses
One of the condensing and dehydrating agents, EDC·HCl, is widely used for polyaniline-carbon nanotube preparation for a cholesterol biosensor, pre-column derivatization of aliphatic amines for HPLC, molecular beacons formation for DNA research, sensor preparation for calcium detection, the fluorescent determination of carboxylic acids, and solid phase micro sequencing of peptides. The by-product generated from the dehydration and condensation reaction with EDC·HCl is easy to dissolve in various solvents, especially water. Since EDC·HCl is less toxic than DIC and DCC, a smooth powdery crystal, EDC·HCl, is readily treated. Therefore, EDC·HCl is an indispensable reagent for the dehydration and condensation reactions in biochemistry, medicinal chemistry, and medicinal chemistry.
Synthesis method

The preparation method of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride comprises the following steps: enabling N, N'-dimethyl-1,3-propanediamine, and carbon disulfide as raw materials to react in an organic solvent to generate an intermediate 1; enabling the intermediate 1 and ethyl chloroformate to react in the organic solvent and preparing an intermediate 2 from triethylamine as an acid-binding agent; enabling the intermediate 2 and ethylamine to react in the organic solvent, to prepare an intermediate 3; adding a catalyst to the intermediate 3, oxidizing one time with an oxidant, toto obtain a crude product 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide, extracting and separating, toto obtain an intermediate 4; and carrying out salt exchange reaction on the intermediate 4 and hydrochloride, toto prepare the product 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride. The method has the advantages of a relatively high conversion rate and a relatively high total recovery rate and is simple to operate and suitable for industrial production.
Reference
[1] Kunihiko Seno. “Determination of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride by Flow-Injection Analysis Based on a Specific Condensation Reaction between Malonic Acid and Ethylenediamine.” Analytical Sciences 25 3 (2009): 389–393.
- Related articles
- Related Qustion
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: Application, Storage, Safety Apr 26, 2023
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, also known as EDC·HCl, is a popular carbodiimide coupling reagent used in bioconjugation chemistry.
- What is 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride? Jul 9, 2020
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide HCl is Commonly known as EDAC, EDC or EDCI, this carbodiimide HCl salt is used as a coupling reagent in the synthesis of amides and carboxylic esters. EDAC is highly soluble in water.
While both PGA and HA are excellent hydrators, PGA has been found to retain moisture even better than HA.....
Apr 16,2024Biochemical Engineering1,8-Diazabicyclo[5.4.0]undec-7-ene, also known as DBU or Diazabicycloundecene, is used as a complexing ligand, a catalyst, and a non-nucleophilic base in organic synthesis.....
Apr 16,2024Organic reagentsYou may like
- The toxicity of Triethylene glycol
May 14, 2024
- Is 1,4-benzoquinone a toxicity compound?
May 11, 2024
- The Synthesis method and Toxicity of 18-Crown-6
May 10, 2024
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride manufacturers
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
-

- $0.00 / 25kg
- 2024-05-24
- CAS:25952-53-8
- Min. Order: 100g
- Purity: 99%
- Supply Ability: 100mt
- EDC HCL
-

- $9.00 / 10g
- 2024-05-11
- CAS:25952-53-8
- Min. Order: 10g
- Purity: min99%
- Supply Ability: 10 tons
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
-

- $50.00 / 1kg
- 2024-04-20
- CAS:25952-53-8
- Min. Order: 1kg
- Purity: 99.10%
- Supply Ability: 5000kg