A semisynthetic pleuromutilin antibiotic: Lefamulin
Jan 5,2024
Description
Lefamulin (Xenleta – Nabriva), a semisynthetic pleuromutilin antibiotic, has been approved by the FDA for IV and oral treatment of community-acquired bacterial pneumonia (CABP) in adults. It is the first systemic pleuromutilin antibiotic to be approved in the US. Lefamulin is active in vitro and in vivo against S. pneumoniae, H. influenzae, M. pneumoniae, C. pneumoniae, Legionella pneumophila, and methicillin-susceptible strains of S. aureus (MSSA)[1]. It has in vitro activity against other streptococcal species, MRSA, Haemophilus parainfluenzae, and M. catarrhalis. Lefamulin is also active in vitro against pathogens that cause sexually transmitted infections, such as Chlamydia trachomatis, Mycoplasma genitalium, and Neisseria gonorrhoeae.
Synthesis method
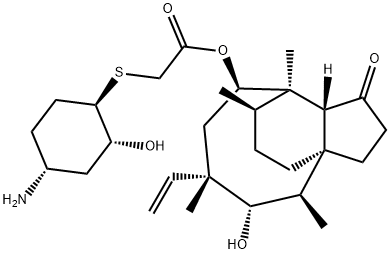
Advantageously, the pleuromutilin cyclooctanol core is a natural product produced by fermentation, reducing the synthetic task to the union of advanced tricyclic fragment 36 with thiocyclohexanol 34[2]. This trisubstituted cyclohexane was produced by first resolving racemic acid 28 with (S)-α- methylbenzylamine (29), shown below. Multiple recrystallizations were performed until the desired optical rotation was achieved, followed by salt breaking with HCl to deliver chiral acid 30. The authors noted that an enzymatic resolution was also viable, but specific details were not described. Curtius rearrangement introduced the amine functionality with complete stereoretention to give Boc-protected amine 31 in 81% yield after chromatography. Exposure of the olefin to mCPBA resulted in synepoxidation of the carbamate. Ring-opening with thiobenzoic acid gave 33 with the regiocontrolled trans arrangement of the S and O substituents as the primary product. The minor regioisomer was removed through crystallization and trituration with toluene/heptane and likely accounts for the mediocre 32% yield of 33 for this step. Hydrazine monohydrate in the presence of dithiothreitol (DTT) converted the thioester to sulfide 34 while minimizing disulfide formation. Although this sequence represents the largest-scale approach, a smaller-scale alternative procedure that avoids chromatography was described in which the steps from acid 30 onward were telescoped, with the amine protected as a trifluoromethyl amide in place of Boc.

The pleuromutilin core 35, obtained by fermentation processes, was tosylated to provide 36, then treated with thiol 34 to give mercaptan 37. Removal of Boc followed by recrystallization gave lefamulin.
References
[1] “Lefamulin (Xenleta) for Community-Acquired Bacterial Pneumonia.” Medical Letter on Drugs and Therapeutics 91 2 (2019): 1709–1710.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
- Related articles
- Related Qustion
The sharing of electrons between O and H is unequal, with the electrons more strongly drawn towards O. This results in an overall polar molecule.....
Jan 5,2024Organic ChemistryPretomanid (also known as PA 824) is a nitroimidazooxazine antimycobacterial drug with a complex mechanism of action.....
Jan 5,2024API