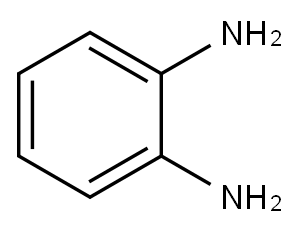
Application and Pharmacology of o-Phenylenediamine
Mar 30,2022
General description
1,2-phenylenediamine appears as colorless monoclinic crystals if pure; technical grade brownish-yellow crystals or a sandy brown solid. Used in manufacture of dyes, photography, organic synthesis.1,2-phenylenediamine is a phenylenediamine in which the two amino groups are ortho to each other. It has a role as a hydrogen donor. It derives from a hydride of a benzene. Phenylethylamine, with the molecular formula c8h11n, has a structural isomer, namely α- Phenylethylamine or 1-phenylethylamine, α- Phenylethylamine has two stereoisomers: (R) - (+) - 1-phenylethylamine and (s) - (-) - 1-phenylethylamine. In human brain, 2-phenylethylamine has the functions of neuromodulatory substances, neurotransmitters and trace amine. Phenylethylamine is a natural compound and can also be found in many foods, such as chocolate, especially after microbial fermentation. It is generally believed that phenylethylamine from food in sufficient quantities will have a mental effect. However, it is quickly metabolized by the enzyme monoamine oxidoreductase, preventing its effective concentration to the brain[1].
Application and Pharmacology
1.FC-99 (C15N2OH18, whose chemical structure was a novel O-phenylethanaminium (1,2-benzenediamine) derivate synthesized in laboratory. In the original studies, FC-99 was scanned by inhibiting LPS-induced NO production in RAW264.7 cells. However, the effect of FC-99 on poly(I:C)-induced TLR3 signal was never tested before. Here we showed that FC-99 exhibited an inhibitory effect on poly(I:C), a synthetic dsRNA-induced inflammatory response and TLR3 expression, we thus assumed that FC-99 could serve as the modulator of TLR3 and be used in the treatment of sepsis. To elucidate the plausible mechanism of FC-99 in sepsis, a septic mice model was introduced by CLP surgery. Our data showed that FC-99 administration significantly reduced the serum level of inflammatory factors, improved multiple organ dysfunction, enhanced bacterial clearance and improved survival. Of note.
2.Phenylethylamine (PEA), also known as β- Phenylethylamine and 2-phenylethylamine, with the molecular formula of c8h11n, are alkaloids and monoamine neurotransmitters, which can increase the level of dopamine in extracellular fluid, inhibit dopamine nerve activation and treat depression. As early as the early 20th century, scientists found through human anatomy that when people's mood changed, the bottom of the diencephalon in the human brain secreted a series of compounds. Such chemical molecules include phenylethylamine and endorphins, which scientists call "emotional hormones".
3.Physical properties phenylethylamine is a colorless liquid at room temperature. Melting point - 60 ℃, boiling point 197-198 ℃, 70-71 ℃ (0.93kpa), flash point 90 ℃, relative density 0.958 (24 / 4 ℃), refractive index 1.5290 (25 ℃). Soluble in alcohol and ether, soluble in water. It smells fishy.
4.Chemical properties: phenylethylamine is strongly alkaline. It can absorb carbon dioxide from the air and form corresponding carbonate. It has fishy smell. Phenylethylamine is a strong base and can form stable hydrochloride crystals. The melting point of the salt is 217 ° C. Phenylethylamine is also a skin irritant and photosensitizer.
Synthesis
1.Simple and efficient synthetic procedures for the preparation of quinoxaline, pyrazine, pyridopyrazine, and benzoxazin-2-one derivatives were developed. The one-pot cascade process involves the acidic elimination of α-aminoxylated dicarbonyl compounds to generate 1,2,3-tricarbonyl compounds and subsequent condensation with 1,4-N,N or -N,O dinucleophiles to afford quinoxaline,pyrazine, pyridopyrazine, and benzoxazin-2-one scaffolds. All the proposed processes do not need extra catalysts, dry solvents, or harsh reaction conditions[2].

Figure1 the rapid and efficient construction of quinoxilines from o-phenylenediamine and α-aminoxylated 1,3-dicarbonyl compounds.
2.M-phenylenediamine plays an important role in chemical raw materials and is mainly used in dyes and organic chemical products. At present, the preparation processes of m-phenylenediamine are mainly iron reduction method and catalytic hydrogenation method. Catalytic hydrogenation method has the advantages of high product recovery, good product quality and low environmental pollution. It is widely used in the production of m-phenylenediamine. The application of m-phenylenediamine is mainly reflected in the preparation of resorcinol, m-aminophenol and new materials. In the future, intermediate phenylenediamine will play an important role in the application of new materials[3].
Safety and Storage
First aid inhalation: move the victim to fresh air and rest in a comfortable place. Ingestion: gargle. Do not induce vomiting. Eye contact: wash carefully with water for a few minutes. If it is convenient and easy to operate, remove the contact lens. Continue flushing. Skin contact: immediately remove / remove all contaminated clothing. Wash skin / shower with water. The contaminated clothes can be reused after cleaning. Call the detoxification center / doctor immediately. Suitable fire extinguishing agents for fire protection: dry powder, foam, fire extinguishing agent not suitable for carbon dioxide: water (likely to expand disaster). Special hazard: be careful, it may decompose and produce toxic smoke under combustion or high temperature.
Specific method: put out the fire from the upwind, and select the appropriate fire extinguishing method according to the surrounding environment. Non relevant personnel should be evacuated to a safe place. In case of fire around: if it is safe, remove the movable container. Special protective equipment for firefighters: when putting out fire, be sure to wear personal protection. The storage method can be stored for a long time in nitrogen atmosphere. Store in a well ventilated place. Keep cool. The storage place must be locked.
References
1.Yan J., Xu Y. & Zhuang F. et al., "Highly efficient synthesis of quinoxaline derivatives from 1,2-benzenediamine and $$\alpha $$ α -aminoxylated 1,3-dicarbonyl compounds," Molecular Diversity, Vol.20, No.2(2016), pp.567-573.
2.Gong W., Hu E. & Dou H. et al., "A novel 1,2-benzenediamine derivative FC-99 suppresses TLR3 expression and ameliorates disease symptoms in a mouse model of sepsis," British Journal of Pharmacology, Vol.171, No.21(2014), pp.4866-4878.
3.Tian Xianfeng, Liu Feng, Liu Guangqin: Research on the application of m-phenylenediamine in synthetic process, China Petroleum and chemical standards and quality, No. 10, 2021, pp. 188-189.
- Related articles
- Related Qustion
- o-Phenylenediamine: applications in fluorescent and colorimetric sensors Jul 12, 2023
o-Phenylenediamine is used to develop sensors for detecting heavy metal ions and organic molecules, offering high sensitivity and selectivity for environmental and health-related applications.
Isoconazole is an imidazole derivative, structurally related to clotrimazole. It has the chemical name 1-[2,4- dichloro-b-[(2,6-dichlorobenzyl)oxy]phenethyl]imidazole nitrate. Isoconazole has a broad spectrum of antifungal activity in vitro....
Mar 29,2022APITranexamic acid, also known as tranexamic acid, tranexamic acid, hemostatic acid, molecular formula is C8H15NO2....
Mar 30,2022APIo-Phenylenediamine
95-54-5You may like
o-Phenylenediamine manufacturers
- o-Phenylenediamine
-

- $0.00 / 25Kg/Drum
- 2024-05-24
- CAS:95-54-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200mt/year
- Lansoprazole Impurity 22
-

- $0.00 / 10mg
- 2024-04-11
- CAS:95-54-5
- Min. Order: 10mg
- Purity: above 98%
- Supply Ability: 10g/month
- o-Phenylenediamine
-

- $40.00/ kg
- 2024-03-31
- CAS:95-54-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000000