Applications of 1,3,4-Oxadiazole
Jan 25,2022
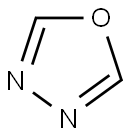
1,3,4-Oxadiazole is a five-membered, conjugated, planar, stable heteroaromatic, comprised of two adjacent nitrogen atoms at the 3,4-positions with one oxygen atom and two vicinal carbon atoms. Each nitrogen atom in the ring is one carbon apart from the oxygen heteroatom. The 1,3,4-oxadiazole ring is electron deficient due to the presence of two pyridine-like nitrogen atoms in the ring and as a result its chemical properties are unlike those of furan as evidenced from the downfield chemical shift of C2(5)H at δ 8.73 ppm (CDCl3) of the parent 1,3,4-oxadiazole. The presence of two electronegative nitrogen atoms deactivates the ring, making electrophilic substitution difficult in the ring at the C2- and C5-positions. From a medicinal chemistry point of view, it is a very important molecule because it is a surrogate of carboxylic acids, esters, and carboxamides and displays a wide spectrum of pharmacological activities.
There are numerous 1,3,4-oxadiazole derivatives with diverse pharmacological activities but some of them are in clinical use as drugs for the treatment of various ailments. Some of the important drugs with the 1,3,4-oxadiazole ring are depicted in the following diagram.
Synthesis of 1,3,4-Oxadiazole
There are a number of general routes for the construction of 1,3,4-oxadiazoles, involving:
1. Oxidative cyclization of azines
2. Cyclization of diacylhydrazines
3. Cyclization of acylhydrazones
4. Ring transformation reactions
5. Miscellaneous reactions
Physical Properties
The parent 1,3,4-oxadiazole is a liquid with a bp of 150°C, is soluble in water, and varies with substituents. The presence of hydrophobic groups decreases the solubility in water. Replacement of the alkyl substituent by the aryl group increases the bp and mp of the compounds.
1H NMR (CDCl3), δ (ppm): C2–H, 8.73; C5–H, 8.73.
13C NMR (CDCl3), δ (ppm): C3, 152.1; C5, 152.1.
Chemical Reactivity
Electrophilic substitution to 1,3,4-oxadiazole is unusual because electron density at the C2-and C5-positions is low. Electrophilic substitution is facile in aryl group present as substituent in lieu of 1,3,4-oxadiazole ring. Nucleophilic substitution reactions are uncommon in 1,3,4-oxadiazoles but ring cleavage is very common.
Applications
Diverse pharmacological activities such as bactericidal, fungicidal, antiinflammatory, sedative, analgesic, antidepressant, antiproteolytic, anesthetic, and anticonvulsant are associated with diaryl- and amino-1,3,4-oxadiazoles. Some of the oxadiazole derivatives such as oxadiazolinones have shown insecticidal and herbicidal activities. One of the oxadiazole derivatives, (2-[4-biphenylyl]-5-[4-tert-butylphenyl])-1,3,4-oxadiazole, is in diagnostic use as a scintillator. 2,5-Disubstituted-1,3,4-oxadiazoles generally produce fluorescence and are used as laser dyes, optical brighteners, scintillators, or electrophotographic photoconductors. 2,5-Dipicryl-1,3,4-oxadiazole has been reported as an explosive. Corrosion produced in mild steel by acid solution has been inhibited by 2-aryl-5-oxadiazoline-5-thiones. A series of liquid crystals based on 1,3,4-oxadiazoles has been prepared and used to study the flexoelectric effect in guest/host mixtures.
- Related articles
- Related Qustion
1,2,5-Oxadiazole is a five-membered, heteroaromatic, planar heterocycle comprised of one oxygen atom with two vicinal nitrogen atoms (N-O-N) and two carbon atoms at the 3- and 4-positions of the ring.....
Jan 25,2022APIDibenzofuran is a tricyclic heterocyclic compound with a 14π electron ring system comprised of two benzene rings fused with 2,3- and 4,5-positions of the furan ring. The chemistry of dibenzofuran has not been extensively studied and explore....
Jan 26,2022Organic reagents1,3,4-oxadiazole
288-99-3You may like