Bromodiphenylmethane: Overview, Applications in Synthesis of N-Substituted Benzamides and Preparation
Apr 2,2024
General Description
Bromodiphenylmethane, a versatile arylalkane compound with unique chemical properties, is instrumental in the synthesis of N-substituted benzamides for potential pain treatment applications. Through selective bromination using an innovative diphenylphosphinite ionic liquid, Bromodiphenylmethane can be efficiently prepared while maintaining high purity. Its role extends beyond organic synthesis, finding applications in various industrial processes. The resulting N-substituted benzamides exhibit diverse structural variations, enabling fine-tuning for optimized pharmacological properties in pain management. This advancement highlights the compound's significance in drug development and underscores the importance of exploring novel chemical entities for effective pain relief therapies. The synthesis process showcases the value of Bromodiphenylmethane and the promising capabilities of the diphenylphosphinite ionic liquid in facilitating targeted reactions, emphasizing their potential in advancing pharmaceutical research and industrial applications.

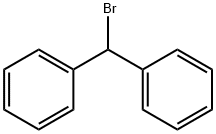
Figure 1. Bromodiphenylmethane
Overview
Bromodiphenylmethane, an arylalkane compound, is characterized by a central methane carbon bonded to two phenyl groups with a bromine atom attached. This unique halogenated aromatic structure grants it distinctive chemical properties. In its solid form, Bromodiphenylmethane ranges from colorless to white and emits a specific odor. While generally stable in standard conditions, it can undergo reactions with oxidizing agents or participate in substitution reactions due to the bromine atom's presence. The synthesis of Bromodiphenylmethane typically involves halogenation reactions. This compound serves various purposes across different domains. It plays a crucial role in organic synthesis, often acting as an intermediate in the production of other compounds. Additionally, it finds applications in specialized industrial processes. Overall, Bromodiphenylmethane's unique structure and reactivity make it valuable in diverse fields, showcasing its significance in chemical research and industrial applications. 1
Applications in Synthesis of N-Substituted Benzamides
Bromodiphenylmethane is a key component in the preparation of N-substituted benzamides, which have demonstrated potential for use in the treatment of pain. As outlined in a invention, Bromodiphenylmethane and its derivatives fall within the scope of formula I, where they can be combined with other reagents and solvents to produce a range of pharmaceutically acceptable salts. The compounds exhibit diverse structural variations, allowing for the adjustment of properties to meet specific pharmaceutical requirements. In the context of pain treatment, these compounds offer promising applications due to their ability to inhibit NaV1.7 activity, a key factor in pain signaling. Furthermore, the flexibility in substituent groups (R1, R', R2, R3, R4, R5, etc.) enables fine-tuning of the compounds to optimize their pharmacological properties for pain management. The synthesis of N-substituted benzamides, facilitated by Bromodiphenylmethane, represents a significant advancement in drug development. The resulting compounds hold potential for addressing unmet medical needs associated with pain, offering new avenues for pharmaceutical research and the eventual improvement of pain management strategies. This innovation underscores the importance of continued exploration and application of novel chemical entities in the pursuit of effective pain relief therapies. 2
Preparation
Bromodiphenylmethane is a compound that can be prepared through a selective bromination reaction using a new diphenylphosphinite ionic liquid (IL-OPPh2) as a reagent and solvent. In this preparation method, the IL-OPPh2 ionic liquid serves as both the reagent and the solvent to facilitate the conversion of alcohols and trimethylsilyl and tetrahydropyranyl ethers into the corresponding alkyl bromides. This reaction takes place in the presence of bromine (Br2) and thiocyanate ions (SCN-) at a temperature of 80°C. One of the key advantages of using this particular ionic liquid is its ability to selectively brominate and thiocyanate alcohols while leaving trimethylsilyl and tetrahydropyranyl ethers unaffected. This selectivity allows for the synthesis of bromodiphenylmethane with high efficiency and purity. Additionally, the use of IL-OPPh2 enables easy separation of the desired bromodiphenylmethane products from the phosphinate byproduct, simplifying the purification process. Overall, the innovative use of the diphenylphosphinite ionic liquid I (IL-OPPh2) offers a promising approach for the preparation of bromodiphenylmethane through a highly selective bromination reaction, demonstrating its potential as an effective reagent in organic synthesis. 3
Reference
1. Bromodiphenylmethane. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 236603.
2. Andrez JC, Chowdhury S, Decker S. Preparation of N-substituted benzamides and their use in the treatment of pain. 2013; Patent Number: WO2013177224.
3. Iranpoor N, Firouzabadi H, Azadi R. A new diphenylphosphinite ionic liquid (IL-OPPh2) as reagent and solvent for highly selective bromination, thiocyanation, or isothiocyanation of alcohols and trimethylsilyl and tetrahydropyranyl ethers. Tetrahedron Letters. 2006; 47(31): 5531-5534.
- Related articles
- Related Qustion
- Bromodiphenylmethane: properties, applications and safety Sep 21, 2023
Bromodiphenylmethane is a white crystalline solid used in the synthesis of Modafinil and O-(triazolyl)methyl carbamate, with safety precautions required.
- Bromodiphenylmethane-Application Dec 27, 2019
Bromodiphenylmethane is a halogenated building block. Benzhydryl group was preferred to the commoner benzyl to protect 2-nitrophenol in the Bartoli (vinyl Grignard) synthesis of 7-hydroxyindole. Protection was by reaction with the phenol in
D-Glucaric acid's diverse applications, aided by metabolic engineering and biosensors, show promise for industry innovations despite challenges in production optimization.....
Apr 2,2024APITantalum(V) chloride has a dimeric structure, versatile reactivity, and health hazards. Its potential in chemistry requires careful handling.....
Apr 2,2024APIBromodiphenylmethane
776-74-9You may like
Bromodiphenylmethane manufacturers
- Bromodiphenylmethane
-
- $100.00 / 1KG
- 2023-12-26
- CAS:776-74-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Bromodiphenylmethane
-

- $1.33 / 1Kg
- 2023-03-21
- CAS:776-74-9
- Min. Order: 1Kg
- Purity: 99%
- Supply Ability: 100000
- Bromodiphenylmethane
-

- $25.00 / 1kg
- 2023-01-17
- CAS:776-74-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000 tons