Carbohydrazide: synthesis and it derivatives biological activities
Jul 4,2023
General Description
Carbohydrazide and their schiff bases hold profound importance as heterocyclic compounds, showcasing their versatility not only in the realm of organic chemistry but also in the domains of physical and inorganic chemistry. These compounds have paved the way for a plethora of bioactive molecules that encompass carbohydrazide moieties and their hydrazone derivatives, which have been meticulously synthesized and thoroughly screened for a multitude of therapeutic properties. Their potential spans across antibacterial, anticancer, antifungal, anti-inflammatory, and numerous other applications, making them a subject of great interest and exploration in contemporary research endeavors. This concise article elucidates several synthetic pathways leading to numerous reported carbohydrazide, as well as their biological activities.

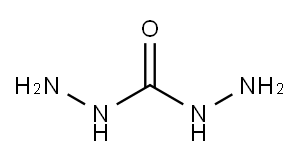
Figure 1. Carbohydrazide
Synthesis
Various approaches have been developed for the synthesis of carbohydrazide, with two methods standing out as the most efficient. One common method involves the preparation of acyl anhydrides and acid chlorides, which subsequently react rapidly with hydrazine to yield the desired carbohydrazide. However, the high reactivity of acid chlorides and anhydrides makes it challenging to control the reaction and stop it after the monoacylation step. The standard procedure for carbohydrazide synthesis is through hydrazinolysis of the corresponding carboxylic esters, which necessitates prior ester formation from the respective acids. Although esters typically generate minimal amounts of diacylhydrazine, less reactive esters may require longer reaction times and/or harsher conditions. Another reported approach involves the use of inclusion complexes of hydroquinone and hydrazine in solid-state hydrazinolysis of esters. Alternative methods directly synthesizing carbohydrazide from acids are inefficient, with only a few examples reported, such as microwave-assisted irradiation or via activated esters formed by catalytic coupling reagents. 1, 2
Biological activities
Anti-inflammatory activities
Several studies reported carbohydrazide derivatives as potential anti-inflammatory agents. To evaluate their anti-inflammatory activity, all synthesized benzoxazole derivatives were compared to diclofenac sodium as the standard over a 4-hour period with 1-hour intervals. The synthesized compounds exhibited the highest activity after 4 hours. A carbohydrazide derivative demonstrated the most potent inhibition, with a percentage inhibition of 47.84%, comparable to the standard's inhibition of 47.84% after 4 hours of administration. Other carbohydrazide derivatives showed inhibitions of 16.26, 30.29, 26.70, 16.26, and 37.30%, respectively. 3
Anti-tubercular activities
The anti-tubercular activities of the synthesized carbohydrazide derivatives were evaluated as follows. Among the copper (II) complexes, their minimum inhibitory concentration (MIC) values against mycobacteria were determined. Complex 79b exhibited the lowest MIC of 1.6 µg/mL against Bacillus subtilis (B.S). Complex 109a had the lowest MIC of 3.9 µg/mL against Staphylococcus aureus (S.A). For Streptococcus pyogenes (S.P), complex 111a showed the lowest MIC of 3.8 µg/mL. Against Enterobacter faecalis (E.F), complex 111b had the lowest MIC of 3.2 µg/mL. Complex 113a demonstrated the lowest MIC of 1.6 µg/mL against Escherichia coli (E.C). Notably, complex 113b exhibited highly effective antimicrobial activity with the lowest MIC of 1.05 µg/mL against Klebsiella pneumoniae (K.P). These results indicate that the synthesized copper (II) complexes displayed significantly enhanced anti-tubercular activity compared to the free carbohydrazide or ligands. 4
Anti-oxidant activities
The antioxidant activities of the synthesized carbohydrazide Schiff base derivatives were evaluated using DPPH as a free radical scavenger. The results demonstrated that the antioxidant activities increased with increasing concentration of the compounds. A study reported the synthesis of a novel Schiff base that incorporated a pyridine moiety and its derivatives for their potential as antioxidant agents. The synthesis involved the hydrozinolysis of ethylpyridine-3-carboxylate to obtain the intermediate pyridine-3-carbohydrazide. Condensation of pyridine-3-carbohydrazide with various aromatic aldehydes resulted in the formation of the Schiff base derivatives containing a pyridine moiety. These findings indicate that the carbohydrazide Schiff base derivatives possess significant antioxidant activity, providing potential for their application as antioxidant agents. 5
Reference
1. Toda F, Hyoda S, Okada K, Hirotsu K. Isolation of anhydrous hydrazine as stable inclusion complexes with hydroquinone and p-methoxyphenol, and their solid state reaction with esters which gives pure hydrazides. J Chem Soc Chem Commun, 1995, 15:1531-1532.
2. Saha, A.; Kumar, R.; Kumar, R.; Devakumar, C. Indian J Chem. 2010, 49B:526-531.
3. Kaur A, Pathak DP, Sharma V, Wakode S. Synthesis, biological evaluation and docking study of a new series of di-substituted benzoxazole derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg Med Chem, 2018, 26(4):891-902.
4. Syed ST, Kannappan G. Schiff Base-Copper(II) Complexes: Synthesis, Spectral Studies and Anti-tubercular and Antimicrobial Activity. Indian Journal of Advances in Chemical Science, 2016, 4(1):40-48.
5. Kumar PP, Rani BL. Synthesis and characterization of new Schiff bases containing pyridine moiety and their derivatives as antioxidant agents. Pharma Chem, 2011, 3(1):155-160.
- Related articles
- Related Qustion
- Carbohydrazide: Biological Properties and Applications in Different Fields Jan 23, 2024
Carbohydrazide displays diverse biological activities and finds applications in solar cells and coordination compounds, showcasing its potential in biochemistry and materials science.
- Synthesis of carbohydrazide Sep 16, 2019
Carbohydrazide which is a volatile oxygen scavenger contributes no solids to the system, reacts readily with oxygen at low temperatures and pressures, and passivates the metal of the boiler system. Carbohydrazide can break down to hydrazine
Carbohydrazide
497-18-7You may like
- Carbohydrazide
-

- $0.00 / 25Kg/Bag
- 2024-05-17
- CAS:497-18-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500mt/year
- Carbohydrazide
-

- $10.00 / 1kg
- 2024-05-17
- CAS:497-18-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 600tons
- Carbohydrazide
-

- $5.00 / 1kg
- 2024-05-17
- CAS:497-18-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 300tons