Clinical application and adverse reactions of baclofen
Aug 3,2022
General description
Baclofen is a γ-aminobutyric acid (GABA) agonist approved for the treatment of spasticity and commonly used in the management of many types of neuropathic pain. Controlled studies have demonstrated the efficacy of this drug in trigeminal neuralgia[1]. Although its precise mechanism of analgesic action is unknown, it is likely that a drug-induced increase in inhibitory activity is sufficient to interrupt the cascade of neural events that culminates in aberrant activity of wide dynamic range neurons, or more rostral neurons in nociceptive pathways, that is the substrate for some types of neuropathic pain[2]. The optimal use of baclofen as an adjuvant analgesic requires an understanding of its pharmacology, side effect spectrum, and dosing guidelines that have proven useful in clinical practice. Failure of baclofen therapy following a prolonged trial requires dose tapering prior to discontinuation due to the potential for a withdrawal syndrome.

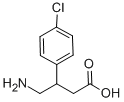
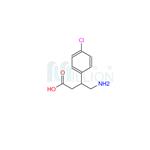
Fig. 1 The structure of baclofen.
Pharmacokinetics of baclofen
Baclofen is rapidly and completely absorbed orally from the gastrointestinal tract. The plasma concentration of baclofen reached its peak 1 ~ 3h after oral administration, but there were large individual differences. The half reduction period is 3 ~ 4h. 70% to 80% of drugs are excreted in their original form in the urine and about 15% are metabolized by the liver. Baclofen may interfere with the release of excitatory neurotransmitters and inhibit synaptic transmission in the spinal cord[3-4].
Pharmacological action
Baclofen can activate GABA β Receptor, which can reduce the reflex potential of single synapse or multiple synapses in the spinal cord and the reflex potential between the posterior root and the posterior root of the spinal cord, and produce skeletal muscle relaxation[5]. Since the 1970s, baclofen has been widely used to treat patients with spinal cord injury spasm. There is no report of using this kind of drug in China. This drug has been approved by the pharmaceutical administration department of the Ministry of health to be used in clinic.
Diseases treatment
Since its introduction in the late 1980s, intrathecal baclofen (ITB) therapy has become the standard treatment for severe generalized spasticity and dystonia in children. Treatment with ITB decreases spasticity in the upper and lower extremities and has been associated with improved function and decreased musculoskeletal contractures[6]. In addition, ITB decreases generalized secondary dystonia and has been associated with improved comfort and ease of care in approximately 85% and with improved function in approximately 33% of patients[7]. Continued effectiveness of ITB in treating spasticity has been observed for up to 17 years, and its effectiveness in treating dystonia has been observed for up to 10 years. Although ITB therapy is frequently associated with complications such as infections, catheter malfunctions, and cerebrospinal fluid leaks, the benefits of therapy appear to outweigh the risks. Additional investigation is needed to determine the effects of ITB on other movement disorders such as athetosis and chorea.
Oral baclofen may be effective in many patients with spasticity, regardless of the underlying disease or severity, and that it is at least comparable with other antispasmodic agents. However, adverse effects, such as muscle weakness, nausea, somnolence and paraesthesia, are common with oral baclofen, affecting between 25% and 75% of patients, and limiting its usefulness[8-9]. Intrathecal baclofen may be an effective alternative as the drug is delivered directly into the cerebrospinal fluid, thus bypassing the blood-brain barrier and thereby optimizing the efficacy of baclofen while minimizing drug-related side-effects. Intrathecal baclofen is a viable option in patients who experience intolerable side-effects or who fail to respond to the maximum recommended dose of oral baclofen.
Pharmacology and toxicology
Baclofen acts as a muscle relaxant in the spinal cord. It inhibits single synaptic and multi-synaptic excitatory transmission, and can stimulate γ -aminobutyric acid (GABA) β receptor and inhibit the release of excitatory amino acids (such as glutamic acid, aspartic acid), thereby reducing excitability, inhibiting the release of nerve cell impulse, and effectively relieving spasm, but to normal nerve muscle[10].
Clinical application
Baclofen, a γ-aminobutyric acid agonist, acts at the spinal cord level to impede the release of excitatory neurotransmitters that cause spasticity. Oral baclofen improves cerebral spasticity mildly, but its activity is limited because of its poor lipid solubility. Cerebrospinal fluid baclofen levels after intrathecal administration are many times higher than those achieved after oral administration[11]. Continuous intrathecal baclofen infusion has been used to treat cerebral spasticity in two patient groups: in older ambulatory children with inadequate underlying leg strength, and in patients with severe spasticity in both the upper and lower extremities. Responsiveness to intrathecal baclofen is confirmed by test injections before insertion of a programmable subcutaneous pump. Continuous intrathecal baclofen infusion dosages vary from 27 to 800 μg/day[12]. Continuous intrathecal baclofen infusion reduces spasticity in the upper and lower extremities, and improves upper extremity function and activities of daily living but has no effect on athetosis in the dosages used to treat spasticity. Complications related to the intrathecal catheter occur in approximately 20% of patients, and infection requiring pump removal occurs in approximately 5%. Preliminary studies indicate that continuous intrathecal baclofen infusion alleviates some forms of generalized dystonia associated with cerebral palsy[13].
Indication
Baclofen is used to improve the spastic symptoms of increased muscle tone caused by pyramidal tract damage, spastic hemiplegia and paraplegia caused by different reasons, such as multiple sclerosis, cerebrovascular disease, spinal cord injury and sequelae of myelitis, cerebral palsy in children, tetanus, intractable hiccup[14-16]. Baclofen can also improve the recurrent vomiting of duodenal obstruction in patients with Duchenne muscular dystrophy, alleviate trigeminal neuralgia and post herpetic neuralgia, and improve the mild improvement of muscle rigidity caused by extrapyramidal damage, such as Parkinson's disease, tardive dyskinesia, and Huntington's disease.
Adverse reactions
Acute baclofen toxicity and withdrawal can present with a constellation of symptoms making differentiation between these two entities and other potential diagnoses challenging[17]. Baclofen withdrawal is associated with numerous complications which may require neurocritical care expertise such as respiratory failure, refractory seizures, delirium, and blood pressure lability.
Long-term use of baclofen when abruptly discontinued can cause withdrawal syndrome, hallucinations, seizures, nausea, constipation, rashes, loss of appetite, and accumulation of intoxication[18]. Dizziness, fatigue, hypotension, nausea, vomiting, euphoria, hallucinations, mental distress, headache, insomnia, tinnitus, numbness, slurred speech, diarrhea, rash, liver damage, tremor. Respiratory depression, coma, convulsions, and sudden withdrawal syndromes may occur during overdose or intrathecal injection[19].
Baclofen use with caution
Baclofen is contraindicated in patients with duodenal ulcer, severe mental illness and epilepsy. The use of this drug can raise blood sugar in diabetics. Additionally, baclofen should be used with caution in patients with poor liver and kidney function and the dose should be reduced. Baclofen should also be contraindicated in patients with urinary retention of hypertonic bladder, and driving should be avoided when taking medication[20]. Older persons with a history of epilepsy, convulsions or mental disorders and cerebrovascular disease should be considered vulnerable to severe adverse reactions to baclofen.
Interaction with drugs
Baclofen instead increases urinary retention. During the period of taking medicine, it is forbidden to drink or share with central nervous system inhibitory drugs[21]. Tricyclic antidepressants can strengthen the relaxation effect of this drug, resulting in severe tension reducing weakness.
Usage and dosage
Take baclofen orally, with the initial dose of 5mg, once a day, and then gradually increase to 5mg, 3-4 times a day. The dosage can be individualized, but it must not exceed 80mg. In addition, children under 10 years old are 0.75 ~ 1mg/kg per day. Children over 10 years old should also gradually add 2mg/kg per day[22-23].
References
[1] M. Antonelli, L. Sestito, C. Tarli, G. Addolorato, Perspectives on the pharmacological management of alcohol use disorder: Are the approved medications effective?, Eur. J. Intern. Med. (2022) Ahead of Print.
[2] N. Bakheet, R. Fouad, A.M. Kassem, W. Hussin, M. El-Shazly, Persistent hiccup: A rare presentation of COVID-19, Respir. Invest. 59(2) (2021) 263-265.
[3] M. Bucholtz, M. Nagy, Polymer vials having standard external dimensions and reduced internal volume, SiO2 Medical Products, Inc., USA . 2022, p. 87pp.
[4] C. Du, Y. Luo, C. Huang, R. Li, Solubility Measurement and Thermodynamic Model Correlation of Baclofen in 12 Pure Organic Solvents, J. Chem. Eng. Data (2022) Ahead of Print.
[5] C.J. Ehret, Y. Almodallal, J.G. Le-Rademacher, N.A. Martin, M.R. Moynagh, A. Rajotia, A. Jatoi, Hiccups in patients with cancer: a multi-site, single-institution study of etiology, severity, complications, interventions, and outcomes, BMC Cancer (2022) Ahead of Print.
[6] Y. Fan, H. Zhang, Y. Yao, Y. Jin, Protective effects of 2OH-saclofen on ultrastructure of amygdala neurons in chronic stress rats, Shenjing Jiepouxue Zazhi 35(6) (2019) 646-650.
[7] R. Fares, T. Flenet, J. Vial, M. Ravaz, V. Roger, C. Bory, S. Baudet, Non invasive jacketed telemetry in socially-housed rats for a combined assessment of respiratory system, electrocardiogram and activity using the DECRO system, J. Pharmacol. Toxicol. Methods (2022) Ahead of Print.
[8] P.R. Fernandes, A. Madnala, N. Donde, A. Pawar, M. Sonawale, Green and efficient synthesis of baclofen, Adv. J. Chem., Sect. B 4(2) (2022) 158-163.
[9] A.T. Gargiulo, P.S. Badve, G.R. Curtis, B.E. Prino, J.R. Barson, Inactivation of the thalamic paraventricular nucleus promotes place preference and sucrose seeking in male rats, Psychopharmacology (Heidelberg, Ger.) 239(8) (2022) 2659-2671.
[10] A.M. Guillory, S.H. Herrera, L.K. Baker, N. Bubula, J. Forneris, Z.-B. You, P. Vezina, B.F. Singer, Conditioned inhibition of amphetamine sensitization, Neurobiol. Learn. Mem. 192 (2022) 107636.
[11] B. Guo, H. Li, R. Wu, L. Xie, Y. Xie, Y. Li, D. Wu, Z. Yue, Effect and mechanism of electroacupuncture promoting Jiawei Shaoyao Gancao Decoction to alleviate spasticity in rats with cerebral apoplexy improve neurotrophic factors and synaptic plasticity protein for better "brain plasticity" in SCA rats, Beijing Zhongyiyao Daxue Xuebao 44(8) (2021) 82-92.
[12] J. Han, J. Escorihuela, S. Fustero, A. Landa, V.A. Soloshonok, A. Sorochinsky, Asymmetric Michael Addition in Synthesis of β-Substituted GABA Derivatives, Molecules 27(12) (2022) 3797.
[13] K. Han, Y. Zhang, X. Wang, X. Xu, T. Luo, Effect of Shaogan Mugua Decoction on neuroethology and regulation mechanism of BDNF/TrkB/CREB pathway in rats with spastic hemiplegia after stroke, Yaowu Pingjia Yanjiu 44(10) (2021) 118-126.
[14] D. Konno, S. Sugino, T.F. Shibata, K. Misawa, Y. Imamura-Kawasawa, J. Suzuki, K. Kido, M. Nagasaki, M. Yamauchi, Antiemetic effects of baclofen in a shrew model of postoperative nausea and vomiting: Whole-transcriptome analysis in the nucleus of the solitary tract, CNS Neurosci. Ther. 28(6) (2022) 922-931.
[15] J. Lu, J. Gu, X. Kong, Isotope-enriched 3-amino-1-propanesulfonic acid derivatives for the treatment of cerebrovascular disease, Risen Suzhou Pharma Tech Co., Ltd., Peop. Rep. China . 2022, p. 121pp.
[16] D.C. Mata, J.F. Davis, Simultaneous quantitative analysis of 39 common toxicological drugs for increased efficiency in an ante- and postmortem laboratory, Forensic Sci. Int. 334 (2022) 111246.
[17] H. Nabipour, M. Mansoorianfar, Y. Hu, Carboxymethyl cellulose-coated HKUST-1 for baclofen drug delivery in vitro, Chem. Pap. (2022) Ahead of Print.
[18] L.K. Nahar, K.G. Murphy, S. Paterson, Baclofen: to screen or not to screen in postmortem blood?, J. Anal. Toxicol. 45(6) (2021) 612-618.
[19] J.V. Pergolizzi, Jr., C. Gharibo, P. Magnusson, F. Breve, J.A. LeQuang, G. Varrassi, Pharmacotherapeutic management of trigeminal neuropathic pain: an update, Expert Opin. Pharmacother. (2022) Ahead of Print.
[20] K. Rurack, K. Gawlitza, V. Valderrey Berciano, V. Perez Padilla, Heterocyclic dye, molecular probe, molecularly imprinted polymer, and sensor assembly, Bundesrepublik Deutschland, Vertreten Durch Den Bundesminister Fuer Wirtschaft und Energie, Germany . 2022, p. 103pp.; Chemical Indexing Equivalent to 178:340907 (DE).
[21] P. Tata, D.S. Alhadeff, A.L. Gomez, J. Schrogie, R.L. Kriel, L. Krach, J.C. Cloyd, G. Diaz, L. Coles, N. Schmitz, Methods of administering intravenous baclofen, Allaysis, LLC, USA; Regents of the University of Minnesota . 2022, p. 39pp.
[22] G. Xu, J. Zhang, Method for synthesis of baclofen from methyl 4-chlorobenzoylformate based on photocatalytic phenyl migration strategy, Lanzhou University, Peop. Rep. China . 2022, p. 9pp.
[23] Y.-q. Yang, L.-x. Liu, X.-y. Hu, L. Shan, L.-y. Yang, X.-l. Wu, F. Gao, H.-z. Li, Prognosis of hereditary spastic paraplegia treated with integrated traditional Chinese and Western Medicine, Chuanbei Yixueyuan Xuebao 36(1) (2021) 93-96.
- Related articles
- Related Qustion
CJC 1295-Ipamorelin is version of a naturally occurring substance that causes release of growth hormone from the pituitary gland.....
Aug 1,2022Biochemical EngineeringXylazine hydrochloride is a non-narcotic drug synthesized in 1962 by Bayer (Leverkusen, Germany), used as a sedative, analgesic, and muscle relaxant in animals.....
Aug 3,2022API