Fondaparinux sodium: Pharmacokinetic Profile and Drug Interaction
Feb 19,2024
General Description
Fondaparinux sodium, a synthetic anticoagulant, showcases a favorable pharmacokinetic profile characterized by rapid and complete absorption, efficient systemic distribution, and predictable metabolism and elimination, primarily via the kidneys. It demonstrates linear pharmacokinetics across various doses and minimal age impact on its distribution and elimination. Additionally, studies reveal no significant pharmacokinetic interactions with warfarin, aspirin, or piroxicam, indicating its safety for coadministration with these drugs. Fondaparinux sodium's high binding affinity to antithrombin III and sustained plasma concentrations make it an effective antithrombotic agent. Its consistent elimination profile and lack of drug interactions further underscore its reliability and versatility in clinical use.

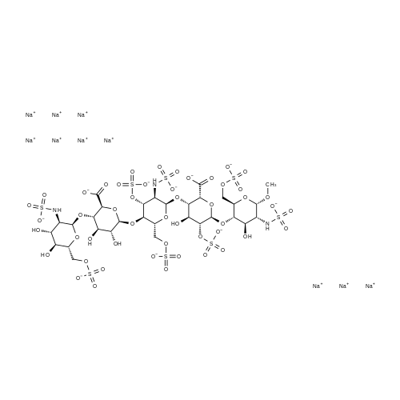
Figure 1. Fondaparinux Sodiun
Pharmacokinetic Profile
Absorption and Distribution
Fondaparinux sodium exhibits rapid and complete absorption when administered subcutaneously at the recommended daily dose of 2.5mg to healthy young male volunteers, achieving a mean maximum plasma concentration (Cmax) of 0.34 mg/L within approximately 1.7 hours. The absorption process reaches half the maximum plasma concentration in just 25 minutes, indicating swift uptake into the bloodstream. The absolute bioavailability of fondaparinux sodium post a 2.5mg subcutaneous dose is 100%, with a corrected volume of distribution of 8.2L, showcasing efficient systemic distribution. Plasma concentrations remain above half Cmax for up to 11 hours after administration, demonstrating sustained presence in circulation. Age has minimal impact on the pharmacokinetics of fondaparinux sodium. In both healthy young and elderly volunteers, the drug's pharmacokinetics appear linear and consistent across different doses, ranging from 2 to 20mg, whether administered subcutaneously or intravenously. The volume of distribution remains relatively stable across age groups and dosages, highlighting the predictable distribution characteristics of fondaparinux sodium. At therapeutic concentrations (≤2 mg/L), it shows high specificity and binding affinity (>94%) to antithrombin III, emphasizing its targeted mechanism of action. These attributes underscore fondaparinux sodium's effective absorption and distribution profile, making it a reliable option for antithrombotic therapy. 1
Metabolism and Elimination
Fondaparinux sodium, a synthetic anticoagulant, is primarily excreted unchanged through the kidneys. Its metabolism and elimination characteristics highlight its renal clearance and plasma clearance rates, alongside its half-life and percentage recovered in urine. In healthy young volunteers receiving a single subcutaneous dose of 2.5mg, the terminal elimination half-life ranges between 13 to 21 hours, with a mean of 17.2 hours. Plasma clearance rates are observed between 0.306 to 0.474 L/h, and renal clearance varies from 0.27 to 0.474 L/h, indicating efficient renal processing. The mean residence time in the body is nearly 24 hours. Studies involving elderly volunteers aged 60 to 85 years showed that pharmacokinetic parameters such as half-life, plasma clearance, and renal clearance remain relatively consistent across different doses, from 2 to 8mg, demonstrating dose independence. Furthermore, the pharmacokinetic profile after intravenous administration mirrors that seen with subcutaneous dosing, with similar half-life and clearance rates. The percentage of Fondaparinux sodium excreted in urine within 72 hours post-dose illustrates effective elimination, ranging from 65% to 77%. These findings underscore the drug's consistent elimination profile, irrespective of administration route or repeated dosing, highlighting its predictable pharmacokinetics. 1
Drug Interaction
Fondaparinux sodium, a synthetic anticoagulant, has been studied for drug interactions with warfarin, aspirin (acetylsalicylic acid), and piroxicam in healthy volunteers, demonstrating no significant pharmacokinetic changes when coadministered. Research involving young healthy volunteers showed that fondaparinux sodium does not interact pharmacokinetically with warfarin. In a study where warfarin was given alongside fondaparinux sodium, there were no changes in the area under the curve (AUC), half-life (t1/2), maximum concentration (Cmax), or time to reach maximum concentration (tmax) of fondaparinux sodium. Similarly, the effect of warfarin on prothrombin time (PT) remained unchanged by the presence of fondaparinux sodium. Another study with aspirin revealed that a single oral dose of aspirin did not alter the pharmacokinetic parameters of fondaparinux sodium, nor did it affect aspirin's capacity to inhibit platelet aggregation when taken together. Additionally, coadministration with piroxicam showed no impact on the pharmacokinetics of fondaparinux sodium or on piroxicam's effects on platelet aggregation and gastrointestinal blood loss. These findings indicate that fondaparinux sodium can be safely administered with these medications without altering their pharmacokinetic profiles or therapeutic effects. 2
Reference
1. Keam SJ, Goa KL. Fondaparinux sodium. Drugs. 2002;62(11):1673-1687.
2. Burggraaff K, Faaij RA, Shoemaker RC, et al Pentasaccharide (fondaparinux, Arixtra®) and the non-steroidal anti-inflammatory drug piroxicam do not interact in healthy subjects. Blood. 2001; 98 (11 Pt. 2): 87b.
- Related articles
- Related Qustion
- Mechanism of action of Fondaparinux sodium Jun 7, 2023
rixtra, also known as Fondaparinux sodium, is a drug that inhibits Factor X, a protein involved in blood clotting.
Favipiravir shows promise in treating COVID-19 by inhibiting virus replication, with effectiveness dependent on a higher dosing regimen than for influenza.....
Feb 19,2024APIEsomeprazole magnesium treats acid-related disorders with specific dosages, requiring caution due to potential severe side effects.....
Feb 19,2024APIFondaparinux sodium
114870-03-0You may like
Fondaparinux sodium manufacturers
- Fondaparinux Sodium
-
- $0.00 / 1Kg/Bag
- 2024-05-23
- CAS:114870-03-0
- Min. Order: 1Kg/Bag
- Purity: 99% up, High Density
- Supply Ability: 20 tons
- Fondaparinux sodium
-
- $0.00 / 1gram
- 2024-03-15
- CAS:114870-03-0
- Min. Order: 1gram
- Purity: 99%
- Supply Ability: 1tons
- Fondaparinux Sodium
-
- $0.00 / 1g
- 2024-02-06
- CAS:114870-03-0
- Min. Order: 1g
- Purity: 99.99%HPLC
- Supply Ability: 200kg