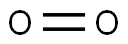How to determine the polarity of oxygen?
Dec 20,2023
Oxygen is a chemical element with an atomic number 8 and symbol O. It lies in group 16 (chalcogen group) of the periodic table and is a highly reactive non-metallic substance. It is an oxidizing agent that forms oxides with multiple compounds readily. There are many known allotropes of oxygen, out of which O2 is the most stable one. It is also known as diatomic oxygen or molecular oxygen and has a significant presence in the atmosphere.
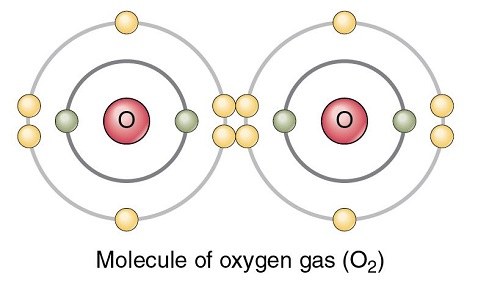
Oxygen polarity
So, is O2 Polar or Non-Polar?
The oxygen (O2) molecule is nonpolar because the molecule is diatomic and both atoms have equal electronegativity. As a result, both atoms share equal charges and there are no partial charges on any atom. Consequently, O2 comes out to be a nonpolar molecule with a zero dipole moment.
Why is O2 nonpolar?
The O2 molecule considered a nonpolar molecule due to the below parameters.
Electronegativity Difference: The electronegativity of a single oxygen atom (O) is 3.44.
When two of these atoms organize themselves with a double bond between them, it forms the structure of molecular O2 (diatomic oxygen). Since both the atoms are the same, the difference in their electronegativity is 0.
Dipole Moment of O2: Both the atoms forming O2 are the same and hence have an equal and opposite effect on each other.
The magnitude of the pull exerted on the shared electrons is equal from both sides, thereby leading to a net-zero force.
So, no charge build-up occurs at any pole and the net dipole moment of the molecular oxygen remains 0 Debye.
Symmetrical shape: The O2 molecule is linear in shape due to the diatomic molecule. Two atoms form double bonds to complete their octet and form a linear geometrical structure.
- Related articles
- Related Qustion
- What is oxygen used for? Is it an element? Mar 4, 2024
Oxygen forms a molecule (O2) of two atoms which is a colorless gas at normal temperatures and pressures.Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium.
- Storage and manufacture of Oxygen Feb 21, 2022
Oxygen is a colourless, tasteless and odourless gas at room temperature and pressure. It supports combustion and, in the correct circumstances, is explosive.
- Oxygen (O) - Chemical properties, Health and the lack of sufficient Oct 29, 2021
Oxygen is a very prevalent and important element and is necessary for sustaining life on this planet. This element is the third most abundant in mass behind helium and hydrogen in the universe. The diatomic form (O2) is the most common pure
PH3 is polar due to a lone pair of electrons with electron-electron repulsion, causing an overall "bent" structure. This results in a dipole moment throughout the molecule.....
Dec 20,2023Inorganic chemistryOleamide is a natural compound found in biological fluids that inhibits gap junction communication by interacting with receptors, and its biosynthesis involves multiple mechanisms.....
Dec 21,2023APIOxygen
7782-44-7You may like
- Oxygen USP/EP/BP
-
- $1.10 / 1g
- 2021-07-29
- CAS:7782-44-7
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min