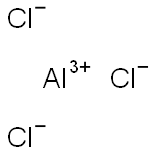
Is AlCl3 ionic or covalent?
Mar 19,2024
Aluminum chloride (AlCl3) is a compound that exhibits characteristics of both ionic and covalent bonding, making it somewhat ambiguous in terms of its classification.
AlCl3 is a compound formed between a metal and non-metal. Al3+ is very polarising due to its high charge density. It is able to pull the electron cloud of chloride to such a great extent that there is orbital overlap between Al and Cl.
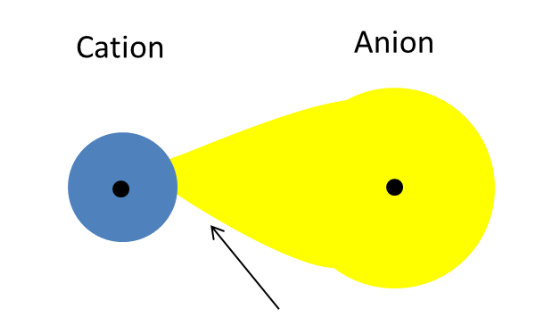
The bond between Al and Cl is covalent and hence AlCl3 is a simple molecule. According to Fajan's rule with increasing size of the anion and with decreasing size of the cation, ionic character of the compound decreases while the covalent character increases.
Here since the cation is same in both the compounds, the size of the anion decides the nature of the compound.
So AlCl3 is covalent.
- Related articles
- Related Qustion
- Why is AlCl3 a Nonpolar molecule? Jan 4, 2024
AlCl3 is a nonpolar molecule because each Al-Cl bond is directed at an angle of 120° to each other in a plane; hence, canceling the dipole moment generated along these bonds is easy.
- Aluminum Chloride: Uses and Solubility Nov 16, 2022
Aluminum chloride is used widely in different fields and freely soluble in numerous organic.
- Introduction of Aluminum chloride Jan 17, 2022
Aluminum chloride is an inorganic substance with the chemical formula AlCl3.
Silicon dioxide is a naturally occurring compound. It consists of silicon and oxygen. The most common form is quartz, which occurs naturally in water, plants, animals and the Earth's crust.....
Mar 19,2024APIAluminum chloride
7446-70-0You may like
- The research on corundum-type Ti2O3
May 10, 2024
- Tungsten nitrides and W-N structures
May 10, 2024
- The structure of Mg2Sn
May 9, 2024
Aluminum chloride manufacturers
- Aluminum chloride
-
- $1.10 / 1g
- 2024-04-17
- CAS:7446-70-0
- Min. Order: 1g
- Purity: 99.0% min
- Supply Ability: 100 tons min
- Aluminum chloride
-

- $9.00 / 25kg
- 2023-12-26
- CAS:7446-70-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000000
- Aluminum chloride
-

- $2.30 / 25Kg/Bag
- 2023-12-12
- CAS:7446-70-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20tons