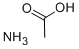Is Ammonium acetate an acid or base and what is the value of pH?
Apr 28,2024
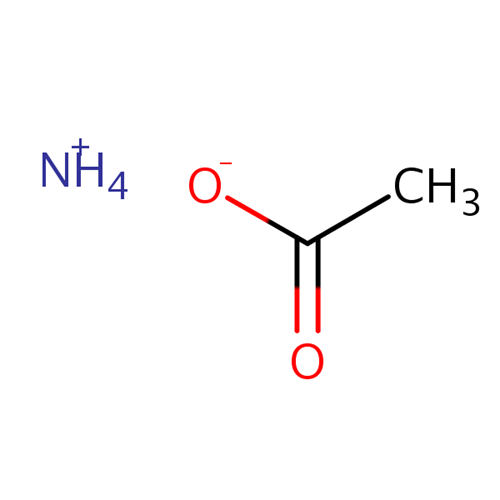
Ammonium acetate is a salt that is a combination of a weak acid and a weak base. When dissolved in water, ammonium acetate has a pH of 7. Ammonium acetate hydrolyses to form ammonia and acetic acid, which dissociate in roughly equal amounts, but this pH is very unstable. Ammonium acetate has a buffering effect at pH values around 4.75 (pKa for acetic acid) and around 9.25 (pKa for ammonium). For this reason, ammonium acetate is commonly used as acetic acid to make buffer solutions. It is a volatile electrolyte that mimics the solubility properties of proteins under physiological conditions. Therefore, it is commonly used as a cell buffer for sample preparation in mass spectrometry.
- Related articles
- Related Qustion
- Learn More About Ammonium Acetate Nov 22, 2022
The passage introduces the physical and chemical properties, uses and hazards of Ammonium acetate.
- How long does 10M ammonium acetate take to dissolve in water? Aug 30, 2019
I need to make 10M ammonium acetate for DNA extraction. I calculated the amount required for 70 ml solution and started dissolving it using a magnetic stirrer. It has been four hours, but the solute hasn't dissolved yet. Is this normal? How
Orthoboric acid(Boric acid) is a natural compound made from oxygen and boron. It is found in water and soil and is used as a food preservative, textile glass fibre, flame retardant, topical wound care agent and insecticide.....
Apr 28,2024APIAmmonium acetate
631-61-8You may like
Ammonium acetate manufacturers
- Ammonium Acetate
-

- $120.00 / 1kg
- 2024-05-09
- CAS:631-61-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Ammonium Acetate
-

- $30.00/ kg
- 2024-03-31
- CAS:631-61-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000000
- Ammonium Acetat
-

- $5.00 / 1KG
- 2024-01-14
- CAS:631-61-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available