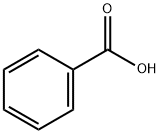
Is benzoic acid polar or nonpolar?
Dec 21,2023
Benzoic acid is an aromatic carboxylic acid. The carboxylic acid (COOH) functional group is attached to the phenyl ring.
Is benzoic acid polar?
Benzoic acid is a polar molecule. The polarity of benzoic acid is accredited to the presence of a polar carboxyl (COOH) functional group in it. The C-O, C=O and O-H bonds present in the COOH group are strongly polar.

A high electronegativity difference of 0.89 units is present between a C-atom and an O-atom in the C-O or C=O bond.
Benzoic acid is considered polar due to the presence of the polar carboxylic acid group, which imparts a significant dipole moment to the molecule. Although the benzene ring is nonpolar, the overall molecule exhibits polarity because of the carboxylic acid group's influence.
Is benzoic acid soluble in water?
Benzene is the main ingredient in benzoic acid, the majority of benzoic acid is nonpolar, making the acid only slightly water soluble. However, its solubility increases as the temperature increases.
Benzoic acid is soluble in hot water. However, it is only sparingly soluble in cold water. Benzoic acid molecules can develop intermolecular H-bonding using their hydroxyl (OH) functional groups.
In summary, benzoic acid is a polar molecule because of a polar functional group (carboxylic acid) which dominates its interactions with other polar substances, such as water, despite having a nonpolar benzene ring as part of its structure.
- Related articles
- Related Qustion
- Is benzoic acid solubility in water? Mar 5, 2024
Benzoic acid is not very soluble in water. It has a high solubility in hot water while poor solubility in cold water. The solubility of Benzoic acid in water increases when the temperature is increased.
- Application and Pharmacology of Benzoic acid Mar 23, 2022
Benzoic acid appears as a white crystalline solid. Slightly soluble in water. The primary hazard is the potential for environmental damage if released.
- What is the difference between benzoic acid and formic acid? Jul 14, 2021
Benzoic acid is also known as benzoic acid. Its molecular formula is C6H5COOH. The simplest aromatic acid in which the carboxyl group is directly connected to the carbon atom of the benzene ring.
Sodium polyacrylate is a superabsorbent polymer, capable of absorbing hundreds of times its own mass in water.....
Dec 21,2023APIMoxidectin is a 16-membered macrocyclic lactone of the milbemycin class. The starting material for the synthesis of the drug is highly functionalized macrolactone nemadectin.....
Dec 21,2023Chemical pesticides ?Benzoic acid
65-85-0You may like
- Hazards to the Environment and Humans of Ethyl Acetate
Nov 16, 2022
- Ethyl acetate - Uses, Properties, Safety etc.
Dec 29, 2021
- What is Trimethyl orthoformate?
Dec 20, 2021
- benzoic acid
-

- $30.00 / 1kg
- 2024-05-01
- CAS:65-85-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- benzoic acid
-

- $1200.00/ T
- 2024-04-28
- CAS:65-85-0
- Min. Order: 20T
- Purity: 99%
- Supply Ability: 5000kg/day
- Benzoic acid
-

- $10.00/ kg
- 2024-04-25
- CAS:65-85-0
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 10000ton