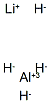Lithium Aluminum Hydride: Lithium Storage Mechanism and its Synthesis Procedure
May 14,2024
General Description
Lithium Aluminum Hydride, a versatile reagent in organic chemistry, is known for its ability to reduce various functional groups. In lithium storage for batteries, XRD analysis reveals a complex mechanism involving phases like Lithium Aluminum Hydride, LiH, metallic Al, and LiAl. Side reactions with electrolytes form new phases and byproducts. The synthesis of Lithium Aluminum Hydride through ball milling is crucial for its purity and electrochemical performance. Evaluation shows a specific capacity of 1729 mAh/g but highlights the need for improved cycling stability. Overall, Lithium Aluminum Hydride's diverse applications and intricate lithium storage behavior underscore its significance in various industries.

Figure 1. Lithium Aluminum Hydride
Overview
Lithium Aluminum Hydride is a versatile reagent widely used in organic chemistry. Lithium Aluminum Hydride is a colorless or gray solid that exhibits a low density of 0.917 g/cm³. Its key properties include stability in dry air and solubility in ethers like THF and diethyl ether. However, Lithium Aluminum Hydride reacts violently with water and alcohols, earning it a hazardous label of 10 for its moisture sensitivity. Lithium Aluminum Hydride is prized for its ability to reduce a wide range of functional groups, including aldehydes, ketones, esters, and more. Additionally, it can induce hydrogenation reactions in olefins and alkynes, making it a valuable catalyst and reducing agent in pharmaceutical, agricultural, and industrial applications. 1
Lithium Storage Mechanism
Lithium Aluminum Hydride is a promising material for lithium storage in rechargeable batteries due to its high theoretical capacity and relatively low cost. Understanding its lithium storage mechanism is crucial for optimizing battery performance. Through XRD (X-ray diffraction) measurements conducted on Lithium Aluminum Hydride samples at different lithium storage stages, the storage mechanism of lithium in Lithium Aluminum Hydride can be elucidated. Initially, as-prepared Lithium Aluminum Hydride exhibits a dominant phase of Li3AlH6 (point A). However, during discharge, a side reaction occurs between Li3AlH6 and electrolytes, leading to the formation of LiH and metallic Al, as evidenced by the detection of diffraction peaks corresponding to these phases at points B and C. Subsequently, as the discharge progresses to point D, a new phase, LiAl, is identified, while Li3AlH6 diminishes. At point E, the fully lithiated products consist of LiAl and LiH. During this process, diffraction peaks resembling those of Al13Fe4 appear, suggesting the formation of Al–Fe intermetallic compounds due to the presence of Fe introduced during the preparation of Li3AlH6 through high-energy ball milling. Furthermore, unknown phases with distinct diffraction peaks are detected during both discharge and recharge processes, indicating the formation of byproducts from the reaction between Li3AlH6 and electrolytes. FTIR (Fourier-transform infrared spectroscopy) analysis further confirms this speculation, revealing absorption peaks corresponding to stretching vibrations of C–O, C–C, C–H, and C–F bonds present throughout the discharge and recharge processes, indicative of electrolyte-derived compounds. In summary, the lithium storage mechanism of Lithium Aluminum Hydride involves the gradual transformation of Li3AlH6 into LiH and metallic Al during discharge, accompanied by the formation of new phases such as LiAl. Additionally, side reactions with electrolytes lead to the generation of byproducts, highlighting the complex interplay between the material and electrolyte components in lithium storage systems. 2
Synthesis Procedure
LiAlH4 is a crucial compound in the synthesis of Li-rich complex metal hydrides, particularly Lithium Aluminum Hydride. The synthesis procedure typically involves a series of steps aimed at achieving a high purity of Li3AlH6 for optimal performance in electrochemical applications. Initially, Lithium Aluminum Hydride is synthesized via ball milling, a mechanical process that involves high-energy milling of LiAlH4 with LiH. This reaction releases hydrogen gas, indicating the formation of Li3AlH6. The efficiency of this step is crucial, as the purity of Lithium Aluminum Hydride directly impacts its electrochemical properties. In the described procedure, approximately 0.44 wt% of hydrogen is released during a 24-hour ball milling process, corresponding to roughly 93% purity of Lithium Aluminum Hydride. Following the synthesis of Li3AlH6, the electrode material is prepared via low-energy ball milling of Li3AlH6 and acetylene black. This step ensures the proper integration of Li3AlH6 with a conductive additive, enhancing its performance as an electrode material. The electrochemical properties of Lithium Aluminum Hydride are then evaluated through galvanostatic discharge-charge measurements. During the first discharge cycle, Lithium Aluminum Hydride exhibits a specific capacity of approximately 1729 mAh/g, corresponding to about 3.5 mol of lithium. However, the initial coulombic efficiency is relatively low, indicating incomplete reversibility of the discharge process. This suggests that further optimization may be necessary to improve the cycling stability of Li3AlH6 electrodes. 2
Reference
1. Lithium aluminum tetrahydride. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 21226445.
2. Liang C, Ye Z, Yang Y, et al. Lithium aluminum hydride Li3AlH6: new insight into the anode material for liquid-state lithium-ion batteries. Heliyon. 2023; 9(11): e21765.
- Related articles
- Related Qustion
- Lithium Tetrahydridoaluminate:A powerful nucleophilic reducing agent Oct 21, 2023
Lithium aluminum hydride is a powerful nucleophilic reducing agent. It reduces almost all functional groups, although isolated double and triple bonds in alkenes and alkynes are generally not attacked.
- Uses of Lithium aluminium hydride in organic chemistry Jan 27, 2022
Lithium aluminium hydride (LiAl H4), commonly abbreviated to LAH, is a powerful reducing agent used in organic chemistry.
- What is Lithium Aluminum Hydride? Dec 13, 2021
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH4. It is a grey solid. It was discovered by Finholt, Bond and Schlesinger in 1947.
Allopurinol is a medication commonly used in the treatment of gout and certain types of kidney stones.....
May 14,2024APIMalic acid, pivotal in food and industry, demands monitoring for quality. Biosensors, especially electrochemical, offer efficient solutions, overcoming traditional method limitations.....
May 14,2024APILithium Aluminum Hydride
16853-85-3You may like
Lithium Aluminum Hydride manufacturers
- Lithium Aluminum Hydride
-

- $30.00 / 1kg
- 2024-06-07
- CAS:16853-85-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20Tons
- Lithium Aluminum Hydride
-

- $1.00 / 1kg
- 2024-02-22
- CAS:16853-85-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000kg/month
- Lithium aluminum hydride in THF
-

- $0.00 / 100kg
- 2024-01-08
- CAS:16853-85-3
- Min. Order: 100kg
- Purity: 5% in THF, 10% in THF
- Supply Ability: 80 tons